Fig. 6.1
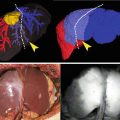
da Vinci® indocyanine green (ICG) near infrared (NIR) fluorescence Imaging Vision System. With permission from Intuitive Surgical, Inc © 2012
There are many fields of application of ICG fluorescence in robotic general surgery, some experimental and still evolving, that include the intraoperative (IO) fluorescent cholangiography to assess biliary anatomy, the evaluation of bowel stump perfusion, the lymph nodes (LN) mapping, and the SLN biopsy in cancer surgery. In addition, ICG fluorescence can be used to endoscopically tattooing the colonic, rectal, and gastric lesions. We describe our experience with current applications of fluorescence in robotic general surgery.
Fluorescent Cholangiography in Single-Site™ Robotic Cholecystectomy
Bile duct injury (BDI) is a serious and feared complication of cholecystectomy: the rate of these lesions passed from 0.2 % at the time of open cholecystectomy [4] to 0.2–0.5 % in the current era of laparoscopic cholecystectomy [5, 6]. In order to better evaluate biliary anatomy and prevent BDI, the adoption of some crucial dissection strategies and the use of a routinary intraoperative cholangiography (IOC) have been recently emphasized [7].
While recognizing the importance of IOC, it has several disadvantages such as a longer operative time, requirement for a multidisciplinary team, staff and patient exposure to radiation and interruption of the regular workflow. Moreover, it is not always easy to interpret an IOC because it provides static images and this can often cause a “misperception” of the biliary anatomy that is considered the primary cause of BDI rather than the lack of skill or knowledge [8]. Thus, as already mentioned by some authors [7], there is a need for a “simpler method of locating the course of the ductal system during the operation, something simpler than cholangiography or ultrasonography.”
The use of the ICG fluorescence imaging system could be a valid solution, as already reported during laparoscopic cholecystectomy [9–11]. ICG NIR-cholangiography is a non-invasive method and it does not require X-rays or bulky equipment such as the C-bow. Moreover, it permits direct real-time IO visualization of biliary anatomy, alternating between white light view and NIR-imaging with a simple switch system.
The abovementioned advantages of the use of ICG fluorescent cholangiography have been recently extended to robotic surgery with promising outcomes, especially during a single site approach [12–14]. The use of the NIR fluorescent vision system, integrated into the Single-Site™ da Vinci® platform has demonstrated to increase safety during single site surgery. This method allows for a safe, real time view of the anatomy of the extra-hepatic biliary tract and a time-efficient dissection of Calot’s triangle.
Technique
A dose of 2.5 mg of ICG is administered intravenously during patient preparation by anesthesia, about 30–45 min before surgery. If fluorescence is not detected in the liver after 60 min, an additional dose of 2.5 mg ICG is again injected intravenously. SSRC starts in the usual fashion [15]. Once the exposure of Calot’s triangle is achieved, the camera view is switched to fluorescence mode for an initial identification of the biliary anatomy (Fig. 6.2). Then, the dissection of Calot’s triangle begins with the incision of the peritoneum and continues as usual, alternatively switching from white to NIR light allowing views of the fluorescent bile ducts in real time. In this way, the surgeon can follow a road map for a safe skeletonization of the cystic duct and the cystic artery, especially in presence of accessory structures (Fig. 6.3). The cystic duct may be clipped under fluorescence before sectioning, especially if it is very short and if there are problems in the biliary confluence. If there are problems with the vascular anatomy during the cystic artery skeletonization, it is possible to proceed with a further injection of 2.5 mg of ICG and, after 10–20 s, obtain a view of the hepatic and cystic arteries and their divisions, and avoid any damage to anomalous branches, especially the branch to the sixth hepatic segment.
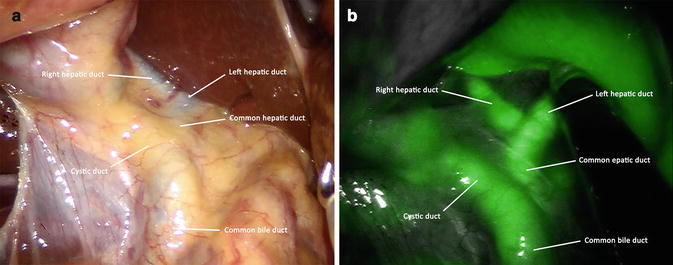
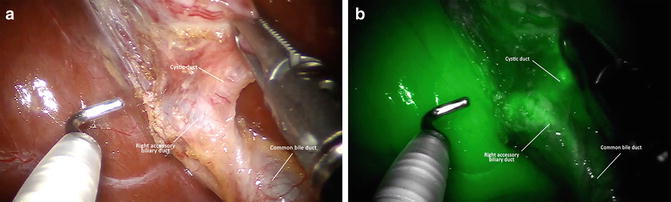

Fig. 6.2
White light (a) and near infrared (NIR) fluorescence (b) view of the biliary anatomy before Calot’s dissection

Fig. 6.3
White light (a) and near infrared (NIR) fluorescence (b) view of a right accessory biliary duct before sectioning the cyst duct
During the detachment of the gallbladder from the liver bed, the use of fluorescence to define the boundary between the gallbladder and liver bed is useful, especially in cases of a thin or an intrahepatic gallbladder, and to visualize any aberrant Luschka ducts (Fig. 6.4). After the procedure, a final fluorescence view of the operative field may be prudent.
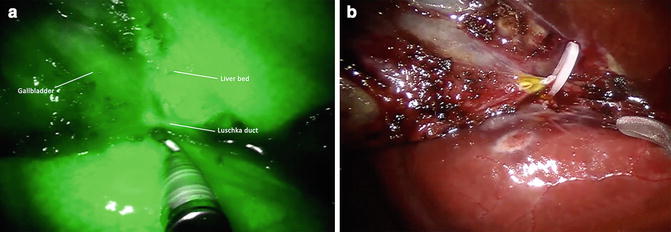

Fig. 6.4
Near infrared (NIR) fluorescence view of Luschka duct before sectioning (a) and white light view of Luschka duct after sectioning (b)
Personal Experience and Considerations
From July 2011 to December 2013, 104 patients underwent SSRC with ICG NIR-cholangiography for symptomatic cholelithiasis and gallbladder polyposis. Five out of 104 patients suffered from acute cholecystitis (4.8 %). Mean BMI was 24.7 kg/m2.
Mean operative time and mean console time were 71 min and 24.1 min, respectively. No statistically significant differences in operative times between standard SSRC (our previous experience) and NIR-fluorescence imaging system SSRC were observed. There were no conversions, BDIs, intra- or postoperative complications or adverse events; mean hospital stay was 1.6 days.
The rates of visualization of the cystic duct, the common hepatic duct, and the common bile duct were 94 %, 71 %, 74 % prior to Calot’s dissection, respectively, and 99 %, 93 %, 98 % after Calot’s dissection, respectively. At least one biliary structure was visualized in 100 out of 104 patients (96 %) before Calot’s dissection, and in 100 % of cases after Calot’s dissection.
The advantages of this method compared to the traditional radiological intraoperative cholangiography are many:
There is no interruption of the regular workflow since images are highlighted on the surgical field during the operation and the surgeon can operate both in white light and fluorescence.
The interpretation of images is simpler because they appear in real time and can be checked with surgical maneuvers of moving structures whilst they are in view. This is in contrast to the traditional IOC images that are fixed on the screen of the radiology equipment with the surgeon working with static information.
It is possible to control the section of the cystic duct with a clear distinction of the bile ducts.
During the detachment of the gallbladder from the liver bed, its wall can be better highlighted and any aberrant Luscka ducts easily found.
The fluorescent bile that usually comes out of the stump of the sectioned cystic duct or of the gallbladder in case of perforation is always clearly visible, therefore the system could potentially highlight any bile leaks. However, this event is not reported in available studies.
If necessary, the vascular anatomy of the hepatic artery and cystic artery can be verified.
C-bow or other equipment is not needed, thus avoiding the un-docking and re-docking of the robotic system.
The procedure does not require any additional time compared to normal SSRC.
The main limitations of ICG-NIR fluorescent cholangiography are obesity and inflammations since fluorescence has the weakness of limited tissue penetration under NIR light [14]. Furthermore, the capability of this method to recognize biliary gallstones or other obstructions is low and of course IOC remains the best method to assess choledocolithiasis.
We can conclude that fluorescent cholangiography is a simple, fast, safe, and effective procedure. It allows clear real-time identification of extra-hepatic biliary anatomy in almost all patients, thus implementing the well-known advantages of SSRC over the traditional single-incision laparoscopic approach. It requires neither interaction of multidisciplinary teams nor additional time, it does not expose patients and staff to radiation and is not burdened by adverse reactions.
Fluorescent Intraoperative (IO) Near Infra-Red Imaging During Colorectal Surgery
The use of fluorescence in robotic colorectal surgery can be useful for some applications currently under development:
real-time identification of vascular anatomy
evaluation of colorectal anastomotic perfusion
lymph node (LN) mapping and sentinel lymph node (SLN) biopsy
tattooing for the localization of colorectal tumors.
Real-Time Identification of Vascular Anatomy
ICG can be easily injected (about 5–7 mg in a bolus) into the blood circulation during colorectal surgery, when blood vessels are exposed, allowing a direct visual observation until its elimination by hepatic clearance in 3–4 min [16]. Because of these characteristics, IO fluorescence imaging is a simple and effective method that helps surgeons to identify vascular structures and so the point section of the bowel, especially in not standardized colic resections, such as resection of the transverse and left colonic angle. Moreover, in case of anatomical abnormalities that can impair blood supply, it is ostensible that this new system could reduce the conversion rate to the open approach. Recently, Bae et al. have underlined the utility of this method in three case reports [17].
Evaluation of Colorectal Anastomotic Perfusion
The perfusion of the intestinal stumps is one of the most important factors to safely perform colorectal anastomosis [18]. The evaluation of the adequacy of stumps’ perfusion is mainly based on some parameters such as presence of an active bleeding from the section line, pulsatility of mesenteric vessels, peristalsis, and the lack of discoloration of bowel segments. These findings are extremely subjective and could represent an inaccurate method to evaluate the risk of anastomotic leak [19, 20].
Many different solutions have been proposed and applied in cases of minimally invasive approaches: laser Doppler flowmetry, visible light spectroscopy, fluorescence laser angiography, narrow band laser imaging techniques, and near infrared reflection spectroscopy [20, 21]. Even if some shortcomings are present, these techniques seem to be feasible methods to evaluate colorectal anastomotic perfusion. More recently, the NIR-fluorescence imaging system of the da Vinci® Si™ HD surgical platform has been introduced and may represent an additional innovative tool.
This device can be used to assess in real time both macroscopic vascular anatomy and perfusion of intestinal stumps. Subsequently, it can support and reassure surgeons in choosing the section point of the bowel during left and right hemicolectomy and anterior resection of the rectum. It can also be useful in non-standardized colonic resections (i.e. transverse colon, splenic flexure), since vascular abnormalities can impair blood supply.
Technique
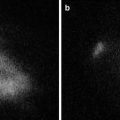
Surgery is progressively carried out with the da Vinci® Si™ HD surgical system following the usual technique of vascular control and preparation of intestinal segments. Then, the optimal point of transection is evaluated and marked by the surgeon with (visible) light. A dose of 5.0–10 mg of ICG is now administered intravenously and after approximately 30–45 s, the surgeon switches to fluorescent vision (Fig. 6.5).
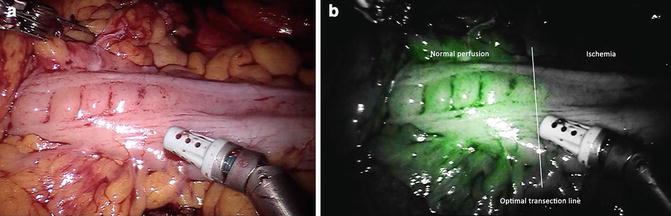

Fig. 6.5
Transection line assessment during left colectomy with white light (a) and near infrared (NIR) fluorescence (b) view
If the site chosen for the section does not appear to be sufficiently perfused, the section line may be revised and the stapler can be moved to a more proximal/distal location according to the best fluorescence reflected point. In case of doubt, the test can be repeated after waiting for a few minutes to allow the dye to washout. However, depending on the tissue, the green fluorescent intensity appears different and at different times.
Regarding the colonic stumps, the vessels of the epiploic appendices and mesentery turn green first, then the green spreads across the intestinal wall. The antimesenteric border of the descending and transverse colon is always a little paler, because the vascularization of the tenia is less intense due to the thickness of muscle tissue. The perfused segments gradually become green until they assume a bright green color, in contrast with the gray segments that are not well vascularized. Further checks may be carried out before and after performing the anastomosis.
With regard to the rectal stump, the pelvic wall turns green first (as does the uterus in women), because it is highly vascularized. After few seconds, the rectal stump colors up allowing the assessment of perfusion at the selected point (Fig. 6.6). The ends of the section lines of the rectum in Knight-Griffen anastomosis or of the colon in latero-lateral anastomosis are often referred to as critical points of leakage for their potential minor irroration: particular attention should be paid to their perfusion.
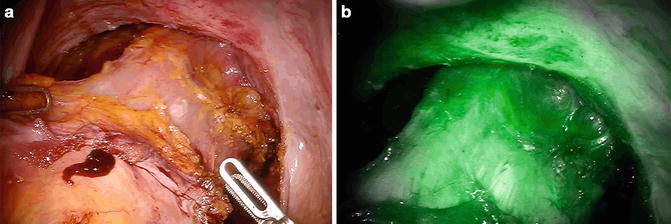

Fig. 6.6
White light (a) and near infrared (NIR) fluorescence (b) view of rectal transaction line during robotic anterior resection. Fluorescence imaging system confirms the good perfusion of the rectal stump
Personal Experience and Considerations
The potential advantages of bowel perfusion assessment using fluorescence system integrated into the da Vinci® system appear interesting but so far only limited clinical data can be found in literature.
Jafari et al. [22] have recently analyzed the effectiveness of NIR-ICG in reducing the rate of anastomotic leak after robotic-assisted low anterior resection (LAR) for rectal cancer in a retrospective case–control study. They compared LAR with and without fluorescence imaging and reported a change in the proximal transection point in 3 out of 16 patients (19 %) and a reduced leak rate of 6 % when compared to 18 % for the control group. Moreover, they described that the overall risk of leak decreased of 12 % in the ICG fluorescence group.
Promising outcomes have been also reported by Bae et al. [17] in two case reports. The authors applied the ICG fluorescence during robotic LAR for cancer to better demark the ischemic area in the distal rectum so that the surgeon was helped to define the distal resection margin. Patients had an uneventful postoperative course.
Hellan et al. [23] have recently published the outcomes of a prospective multicenter study evaluating the impact of fluorescence imaging on visualization of perfusion and subsequent change of transection point during left-sided robotic colorectal surgery. Fluorescence imaging was applied on 40 patients resulted in a change of the proximal transection location in 40 % (16/40) of the candidates: two patients (5 %) with a change in transection line developed an anastomotic leak at postoperative days 15 and 40. Authors conclude that fluorescence imaging provides additional information during determination of transection line in left-sided colorectal procedures and this results in a significant change of transection location, particularly at the proximal transection site.
From September 2011 to December 2013, we have performed 100 full-robotic multiport colorectal resections for benign and malignant diseases (unpublished data) using fluorescence for bowel stump evaluation: 40 right colectomies, 30 low and ultralow anterior rectal resections, 25 left colectomies, and 5 splenic flexure resections. We routinely chose the transection line, after mesenteric section and bowel preparation, according to intestinal perfusion as shown by fluorescence imaging system. There were no intra-operative or anesthetic complications associated with the injection of ICG dye and fluorescence bowel assessment was easily accomplished in real-time in 100 % of the cases with no additional operative time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree