Fig. 14.1
Connections via s-video cable between Aloka ProSound Alpha-7 and da Vinci S
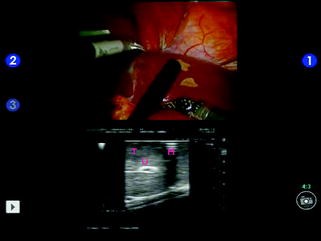
Fig. 14.2
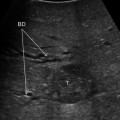
Split-screen display mode using a traditional laparoscopic transducer; tumor (T); umbilical portion (U); resection margin marked with the bipolar forceps (M)
BK Medical has recently placed on the market an ultrasonograph equipped with a laparoscopic probe that can be manipulated by the robotic graspers (ProGrasp Forceps, Intuitive Surgical Inc., Sunnyvale, CA) (Fig. 14.3). The ProART Robotic Transducer Type 8826 is used together with a Flex Focus 800 Ultrasound system, which can be operated by wireless remote control UA1237 for use with the da Vinci Surgical System. The probe has a fin on its back that can be caught with ProGrasp forceps and manipulated by the console surgeon. The Flex Focus 800 is connected to the da Vinci Si console with a DVI cable for integration of picture-in-picture images with TilePro and can be remotely adjusted by the console surgeon. A similar system has been provided by Hitachi Aloka. A second solution has been tested in the preclinical setting. A prototype of a new robotic device for LIOUS has been developed by collaboration between the Johns Hopkins University and Intuitive. The device is composed by a linear transducer installed on an articulated probe controlled from the master tool manipulators of the surgical console [8]. In preclinical trials the probe was used with dedicated open-source software enabling to display the US images in different ways. The “split screen” display mode in which the surgeon sees the endoscopic and US views side-by-side. The “picture-in-picture” display mode that insets the US image into the endoscopic view and finally the “flashlight” display mode in which the US image is overlaid onto a 3D representation of the imaging plane in the stereo view of the console. The effect of this mode is to display the US image in the plane in which it is physically acquired by the transducer. Image magnification and camera filtering are other evolutions of the robotic view system with interesting future clinical applications. The key factor of this evolution is that the digital image at the console can be easily manipulated and the camera can be adapted to be sensible to different frequencies of light. Intuitive has recently cleared to the European market new system components including a surgical endoscope capable of visible light and near-infrared imaging. The 3D HD stereoscopic camera provides visible light and near-infrared illumination through the surgical endoscope via a flexible light guide. The system allows surgeons to view high-resolution near-infrared images of blood flow in vessels and microvessels, tissue and organ perfusion, and biliary excretion in real time during minimally invasive surgical procedures. The da Vinci robotic system with this new camera has been just adopted in clinical studies to replace intraoperative cholangiography using indocyanine green dye in order to delineate biliary anatomy and to detect bile leaks on the resection surface [9].
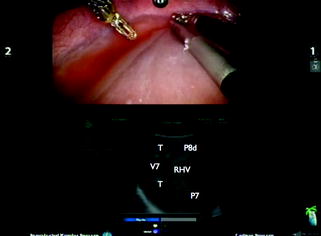

Fig. 14.3
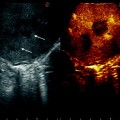
Split-screen display mode using the BK transducer; tumor (T); portal branch for segment 8 dorsal (P8d); right hepatic vein (RHV); hepatic vein for segment 7 (V7); portal branch for segment 7 (P7)
14.4 Operating Room Setup, Patient, and Trocar Position
Robot docking and patient position are of paramount importance to easily approach all the different liver segments for two motivations. Actually, the cart is heavy and once the robotic arms are connected to the trocars, changing position and operating field is relatively challenging. Second, a proper patient position is necessary to take advantage of gravity and the weight of the liver to improve exposure. Therefore, position of all the devices in the operating room, trocars, and patient has to be individualized according to the tumor location. When approaching anterior segments and segment 1 (Spiegel lobe) a reversed Trendelemburg supine patient position is optimal. The on-table surgeon stands between the legs and the scrub nurse and instruments are positioned lateral to the left leg (Fig. 14.4). The trocars are generally positioned along a bowl-shaped line passing through the umbilicus (Fig. 14.5).
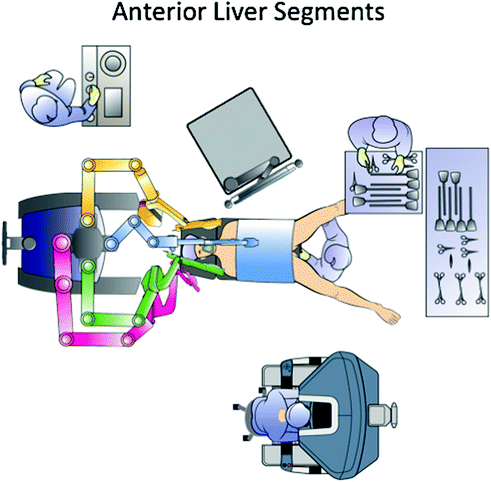
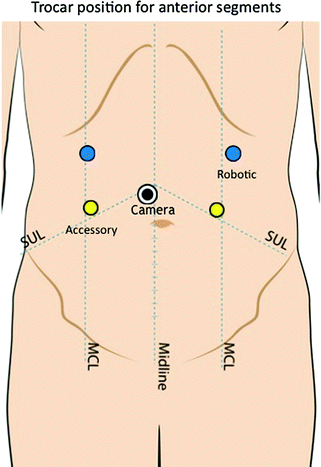

Fig. 14.4
Operating room setup for liver resections in the anterior segments

Fig. 14.5
Trocar position for liver resections in the anterior segments
For lesions located in the right posterolateral sector (upper segment 6 and segment 7) the patient is rotated on the left flank in order to facilitate liver mobilization and inferior vena cava dissection, when necessary. The camera port and the left-sided trocars should be placed as close as possible to the right costal margin, whereas the right trocar can be inserted in the intercostal space between the 10th and 11th rib close to the scapular line. At this level, the risk to accidental injury of the lung is very low and direct access to the posterolateral segments is provided (Figs. 14.6, 14.7, 14.8) [10]. For bilobar liver metastases with involvement of both anterior segments and S7-8 two options are equally effective; redocking the robot and changing patient position according to the tumor location; and using a semi-left lateral patient position with the robot over the patient’s head (Fig. 14.9). The latter is preferred in our institution since it does not require robot redocking and changes during surgery of patient position with possible contamination of the surgical field.
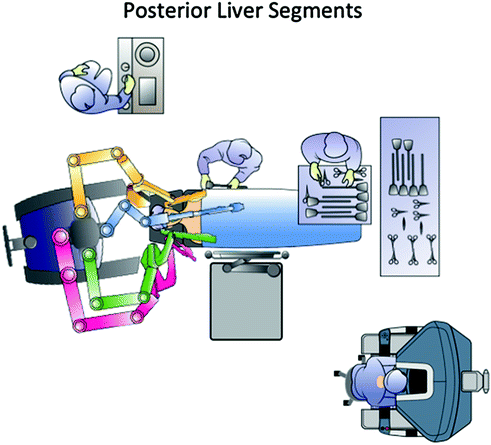
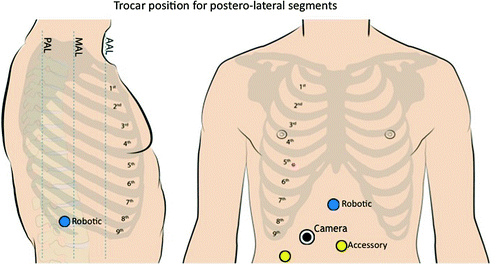
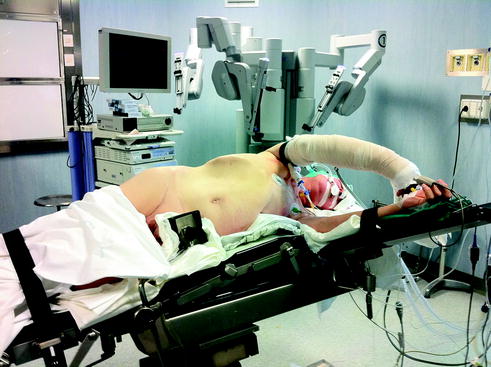
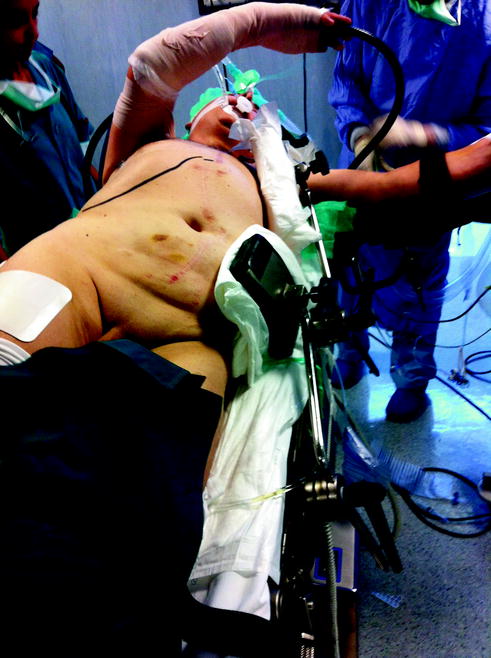

Fig. 14.6
Operating room setup for liver resections in the posterior segments

Fig. 14.7
Trocar position for liver resections in the posterior segments

Fig. 14.8
Left lateral position for lesions in upper S6 and S7

Fig. 14.9
Semi-left lateral patient position for bilobar lesions
14.5 Ultrasound Exploration
Ultrasound exploration follows the same general rules of traditional laparoscopic liver surgery. The typical frequency range adopted for LIOUS is 5–10 MHz. Access for the US probe to the peritoneal cavity is achieved through 10-or 11-mm ports which are usually the same ports used by the on-table surgeon for suction/irrigation and retraction (Figs. 14.5, 14.7). The supramesocolic space is instilled with saline solution until the space between the liver dome and the right diaphragm is completely filled in order to better explore the bare area of S7-8 and the hepatocaval confluence. A first ultrasound exploration of the liver is performed before the robot is docked and any other maneuver on the liver has been performed to avoid artifacts. This first exploration is intended to allow the surgical staff acquiring a clear 3D picture of liver vascular anatomy and lesion location. The examination is usually performed by contact scanning using the liquid film on the glissonian surface as a coupling agent. The hepatocaval confluence is usually visualized with the probe introduced through a right port and placed on S4a. Moving the probe between the diaphragm and the liver dome, the right hepatic vein is then identified. Portal bifurcation and right hepatic pedicles are visualized positioning the transducer on S4b and moving it to the right side. Left portal pedicles and left hepatic vein are better identified from the left upper quadrant. Using the linear probe, exploration of the diaphragmatic margins of S7-8 is carried out placing the probe on the water surface created in the right subdiaphragmatic area (Figs. 14.10, 14.11). Finally, the transection plane is outlined on the liver capsule with monopolar diathermy. When the robot is docked and resection begins, a continuous and meticulous ultrasound assessment of transection margin is imperative due to the lack of tactile feedback. Using a traditional laparoscopic US probe, the on-table surgeon checks the direction of the transection plane while the console surgeon can view on the screen the real-time ultrasonography and suggest to the on-table surgeon modifications of the probe position. Otherwise, the console surgeon has to move to the table to do it by him/herself. With the more sophisticated methods described above, the console surgeon can manipulate both the probe and US system during all the time of parenchymal transection. Concerning US-oriented anatomical resections for HCC, the two techniques described to identify the boundaries of liver segments were reproduced in minimally invasive surgery only in selected cases. The new generation laparoscopic US transducers—including those for robotic surgery—have a groove for needle guidance that allows performing the Makuuchi puncture technique. The “finger compression” described by Torzilli is more challenging to reproduce. The linear shape of the laparoscopic transducers, the scarce pressure applicable with the laparoscopic tools, thickness and stiffness of the liver are all factors that hamper laparoscopic compression of intrahepatic portal branches. Our personal experience is limited to segments 4b and 6 but current technological deficiency does not rule out the possible evolution of new devices to reproduce this technique in minimally invasive surgery (Fig. 14.12).
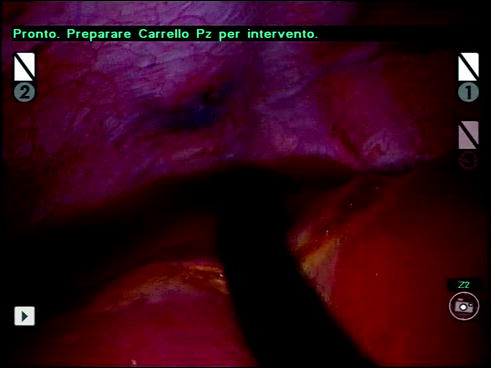





Fig. 14.10
LIOUS exploration of S7 before liver mobilization
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree