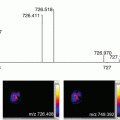
Fig. 1.
(a) Stereotaxic frame used to freeze the carcass while maintaining spinal alignment. (N.B. the array of holes in each side permits the free flow of the hexane/dry ice freezing solution to the carcass). (b) The blocking mold and cryomacrotome stage , which combine to form the freezing mold (c)
Forceps—12″ surgical forceps or similar to remove the animal from the hexane/dry ice bath.
CMC—a 2% w/v solution in water prechilled to 4 °C.
Metal mold suited to the cryomacrotome large enough to fit the specimen. The mold shown in Fig. 1b, c combines the mold with the Leica stage in a single unit. Another option is a metal mold where the sample block is attached to the cryomacrotome stage with additional CMC after initial sample freezing.
2.2 Known Concentration Standards (If Analysis of Exogenous Compounds Is Required)
- 1.
Standard curve/dilution series of the compound of interest in a suitable biological matrix, e.g., tissue homogenate. This should include a blank (tissue only) sample as well as four to eight samples at a concentration range from the lower limit of quantitation of the instrument to above the highest expected concentration.
- 2.
CMC block with suitable sized holes drilled for each standard.
2.3 Cryo-Sectioning (See Note 1 )
- 1.
Specialized whole-body cryomacrotome similar to a Leica CM3600 XP (Leica microsystems, Wetzlar, Germany).
- 2.
Macro-Tape-Transfer-System (Instrumedics Inc., St. Louis, MO, USA). If this system or similar is not available adhesive tape can be used and directly mounted to the target plate.
- 3.
Small rubber hand roller.
2.4 Mounting
- 1.
Clean, thin aluminum plate (similar in thickness to TLC plates)/MALDI target plate or glass slides precoated with the UV polymerising adhesive (part of the Macro-Tape-Transfer-System).
- 2.
Sample holder suited to the mass spectrometer.
- 3.
Double-sided tape.
- 4.
Lyophilizer.
2.5 MALDI Matrix Coating
- 1.
Sonic bath.
- 2.
MALDI matrix 50 mL at 25 mg/mL αCHCA (α cyano-4-hydroxycinnamic acid) in 70:30 (v/v) ethanol:water (0.1% TFA) (see Note 2 ).
- 3.
Pneumatic air spray gun with compressor (Iwata-Media Inc. OR, USA) or similar.
- 4.
Heavy isotope labeled internal standard, if available, should be added to the matrix at a concentration that is similar to the expected analyte concentration and within the instrument’s limits of detection (determined for the analyte of interest in suitable sample matrix).
2.6 MS Setup and Data Acquisition
- 1.
Mass spectrometer with MALDI source. (Instrument setup varies between manufacturers: in the example given here a MALDI Synapt HDMS (Waters Corporation. Manchester, UK) Q-oaTOF MS is used.
- 2.
A flat-bed scanner.
- 3.
Positive ion calibration solution polyethylene glycol (PEG): 1 mg/mL PEG 600, 1000, 1500, and 2000 (average molecular weight), with 0.5 mg/mL sodium iodide in 2 mg/mL αCHCA in 50:50 water:acetonitrile (v:v).
- 4.
Negative ion calibration solution PEG sulfate. Prepare a 1:10,000 (v:v) dilution of PEG sulphate in 2 mg/mL αCHCA in 50:50 water:acetonitrile (v:v).
2.7 Data Analysis
- 1.
Depending on instrument manufacturer data analysis may vary. Most data can be converted into files readable by BioMap (Novartis, Basel, Switzerland) [29]. BioMap is freely available software suitable for MALDI image analysis and can be downloaded from www.maldi-msi.org.
3 Methods
3.1 Snap-Freezing (45 min)
- 1.
Prepare the hexane/dry ice bath by half-filling a large rectangular bowl with hexane (size depends on sample; bowl must be large enough to contain hexane/dry ice solution and allow the sample to be fully submerged), then carefully adding dry ice pellets to the hexane (CARE, see Note 3 ). The amount of dry ice pellets required will vary depending on size of the bowl and ambient temperature, etc. Dry ice should be added until the solvent is cold and some pellets of dry ice remain floating in the hexane.
- 2.
The euthanized rodent is placed in a frame to ensure alignment before freezing (Fig. 1a). If a frame is not available, gently pulling the head and tail in opposite directions to get the spinal column in line using forceps will achieve a similar outcome. Pinning to a cork board can also be used if required.
- 3.
While maintaining the correct alignment submerge the rodent in the hexane/dry ice bath until completely covered. Fully freeze the sample; the time required varies depending on sample size. For instance, a typical male Sprague-Dawley rat (ca. 260 g) should be frozen for 30 min.
3.2 Blocking in CMC (30 min)
- 1.
The freezing mold with stage (Fig. 1b, c) is precooled by placing on a bed of dry ice; a small amount of prechilled CMC is added to the bottom of the mold. The frozen carcass is then placed into the center of the container and the surrounding area is filled with prechilled (4 °C) 2% CMC (w/v). Ensure the container is at least three-quarters submerged and leave in the hexane/dry ice bath for 30 min or until fully frozen (the dry ice will evaporate over time and will need to be topped up as required). Once frozen the sample can be removed from the container and stored in a sealed bag at −80 °C until required.
3.3 Addition of Standards (Quantitation)
- 1.
Relative quantitation of exogenous compounds (e.g., pharmaceutical agents) can be achieved using blood or tissue homogenates spiked with the compound of interest at concentrations covering the expected range. This standard curve can be added to a CMC block by drilling holes in the block and filling these with spiked homogenates and freezing in the hexane dry ice bath. (N.B. at this stage the sample can be stored in a sealed bag at −80 °C until required).
3.4 Preparation of Mounting Media
- 1.
- 2.
Coat the mounting plates with the UV curable adhesive supplied with the Macro-Tape-Transfer-System (Instrumedics Inc., St. Louis, MO, USA) following the manufacturer’s instructions. Skip this step if using direct tape mounting without a tape transfer system.
3.5 Cryo-Sectioning (All Equipment and All Steps Are Completed Inside the Cryotome Chamber)
- 1.
For whole-body sectioning a specialized cryomacrotome is required (e.g., Leica CM3600 XP); smaller samples can be sectioned using a standard cryomicrotome.
- 2.
Securely mount the block into the holder in the cryomacrotome, ensuring the correct orientation for sectioning (sagittal/coronal).
- 3.
Using a fresh blade (care: the blade is extremely sharp) for each sample start to trim in the sectioning using large steps 50–100 μm until the required tissue depth is reached.
- 4.
Set the cryomacrotome to 30 μm section thickness; cut and discard the next section.
- 5.
Apply the adhesive tape to the top of the sample block and ensure it is firmly adhered using a rubber or foam hand roller.
- 6.
Slowly cut the section while applying slight pressure a few millimeters in front of the cutting blade to prevent the tape lifting off the tissue. When cutting the section gently lift the tape with the attached section as it is being cut, ensuring not to pull the tape because the section can easily be damaged.
- 7.
Cutting a second serial section for histological staining can be very useful for comparison with the MALDI image and will provide cellular information. (This section should be mounted to a glass slide for staining.)
- 8.
Repeat steps 3–7 for all the tissue levels required.
- 9.
If using direct tape mounting stick the sample tissue side up directly on the target plate using double-sided tape (skip steps 10–12).
- 10.
Place the tissue section face down onto the precoated mounting medium, ensuring good adhesion using a hand roller.
- 11.
Following the manufacturer’s instructions polymerize the adhesive using a pulse of long wave UV light .
- 12.
Carefully remove the tape from the tissue section, leaving it firmly attached to the mounting medium.
3.6 Freeze Drying/Lyophilizing
- 1.
Remove the section(s) from the cryostat chamber along with a metal block at least the size of your sample and place directly in the freeze-drier chamber. The metal block prevents the section(s) from thawing between the transfer and the start of the freeze drying process (the very thin tissue section would rapidly thaw if not kept on a frozen metal block).
- 2.
Immediately evacuate the freeze dryer and dry the section for 45 min. (Fr4eeze-dried sections can be stored in an air-tight container or bag at −80 °C until required. However, when defrosting before use ensure the container/bag remains sealed until the sample has reached room temperature to prevent condensation forming on the tissue section).
3.7 MALDI Matrix Coating
- 1.
Prepare the MALDI matrix solution (Subheading 2.5, item 2) and sonicate for 15 min before use (see Note 5 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree