Radiological guided intervention techniques are discussed in obstetric and gynecologic patients. Fallopian tube recanalization, postpartum hemorrhage control, techniques of treating uterine leiomyomas, pelvic congestion treatment, and the use of percutaneous and transvaginal ultrasonography-guided aspirations and biopsy are covered. These techniques use basic radiological interventional skills and show how they are adapted for use in the female pelvis.
Key points
- •
Uterine artery embolization is an effective means for the treatment of postpartum hemorrhage of several causes, including atony and invasive placenta.
- •
Ultrasonography-guided intervention can be used in daily practice in the diagnosis and treatment of benign and malignant gynecologic conditions.
- •
Clinicians should be familiar with the clinical presentations, procedural steps, and outcomes of high-intensity focused ultrasonography, fallopian tube recanalization, and pelvic congestion.
- •
Interventional radiology can be used for the treatment of pelvic congestion syndrome, uterine fistula, and uterine leiomyomas, and for fallopian tube recanalization.
Introduction
This article introduces some of the many techniques of imaging-guided intervention that are available in the pelvis. Many facets of obstetric and gynecologic disorders from assisted fertility, through biopsy or aspiration of adnexal masses to embolization of postpartum bleeding, are considered. The techniques are often common to nongynecologic applications but this article provides an aide memoire to how they can be used in the female pelvis.
Fallopian Tube Recanalization
Infertility is a common problem that affects many couples. According to the Centers for Disease Control and Prevention, infertility affects 6.1 million or 10% of women of reproductive age in the United States. Fallopian tube obstruction contributes to one-third of cases of infertility, with 10% to 25% of these cases attributed to proximal fallopian tube obstruction. , The unique anatomy of the proximal fallopian tube, with its muscular wall, small diameter, and tortuosity, makes this segment prone to spasm, mucus plugging, and scarring from inflammation leading to obstruction and infertility. The American Society for Reproductive Medicine recommends fallopian tube recanalization (FTR) as a treatment of infertility in women with proximal tubal occlusion.
FTR is a minimally invasive and cost-effective alternative to in vitro fertilization (IVF) with the added advantage of allowing women the ability to conceive naturally, at their own pace and without hormonal intervention. FTR is a well-established and well-tolerated procedure with similar outcomes to IVF.
Preprocedural planning
This minimally invasive procedure is performed in an ambulatory setting, during the follicular phase of the menstrual cycle, before ovulation. Preprocedural prophylactic antibiotics such as doxycycline 100 mg twice a day is recommended 2 days before FTR. The procedure is routinely performed with moderate sedation. Postprocedural pain and cramping are predominately managed with nonsteroidal antiinflammatory drugs (NSAIDs). Following FTR, patients can attempt to conceive soon after the procedure.
Fallopian tube recanalization procedure
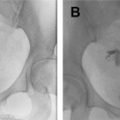
FTR is performed using a sterile technique with the patient in the lithotomy position. The procedure is composed of 3 components: uterine access, hysterosalpingogram (HSG), and tubal recanalization. Uterine access is performed using a balloon occluding sheath or catheter. Next, the HSG is performed with slow injection of a dilute nonionic contrast agent to localize the uterine cornua ( Fig. 1 ). Once proximal fallopian tubal obstruction is confirmed, selective catheterization of the blocked tube is performed using a 5-French or 3-French coaxial catheter system ( Fig. 2 ). A hydrophilic guidewire is used to cross the obstruction, followed by selective tubal injection (salpingogram) to confirm patency ( Fig. 3 ). A postrecanalization HSG can also then be performed ( Fig. 4 ). In light of recent evidence showing higher rates of pregnancy and live births following HSG with oil-based contrast, a small amount of oil-based contrast can be injected following confirmation of tubal recanalization.




Clinical outcomes
The technical success rate of this procedure is in the range of 70% to 90%, and patency rates are reported to be over 60% for up to 1 year after recanalization, and reocclusion of the fallopian tube can be treated with repeat recanalization. The pregnancy rates following the procedure are in the range of 30% to 60%.
Adverse events
The minor complications of FTR include mild uterine cramping and vaginal bleeding 1 to 3 days following recanalization. Complications of tubal perforation, ectopic pregnancy, and adnexal infections have been reported but are rare. Radiation doses to the ovaries from this procedure are less than 1 rad (10 mGy), with a mean dose of 2.7 mGy and patient effective dose of 1.2 mSV. , ,
Postpartum hemorrhage
Postpartum hemorrhage (PPH) occurs in 6% of all deliveries. PPH is defined as a blood loss greater than 500 mL within 24 hours after vaginal or cesarean delivery that requires specific administration of oxytocin, which is a first-line uterotonic drug. Severe PPH is a life-threatening condition with hemodynamic instability that requires the use of prostaglandins; uterine tamponade; and, in case of failure, uterine artery embolization (UAE) or surgery. Severe PPH represents 1.86% of all deliveries. PPH that occurs less than 24 hours after delivery is also called primary or early PPH. When PPH occurs more than 24 hours but less than 6 to 12 weeks after delivery it is called secondary or late PPH.
Uterine atony and trauma or laceration of the lower portion of the genital tract are the main causes of primary PPH, occurring in approximately 80% and 5% of PPH, respectively. Other less frequent causes of primary PPH consist of abnormally invasive placentation, congenital or acquired coagulation disorders, uterine rupture or inversion, bladder flap hematoma, retention of blood clots or placental fragments, and leiomyomas. Retained placenta, abnormally invasive placentation, uterine subinvolution, coagulopathies, and ruptured pseudoaneurysm represent the main causes of secondary PPH. , Usually, pseudoaneurysms occur on uterine arteries after cesarean section and on cervicovaginal arteries after vaginal delivery.
The overall success rate of UAE in women with PPH approaches 91%. , , Predictive factors of failed UAE include disseminated intravascular coagulation, , transfusion of 5 to 10 packed red cells, blood loss greater than 1.5 L, , severe arterial vasoconstriction on angiography, and abnormally invasive placentation. , Repeat UAE when the first uterine embolization session has failed has a success rate of 67% to 80%. , , Of note, active bleeding during angiography has been suggested as a predictive factor of repeat UAE.
Complications of UAE occur in 3% of women with PPH. They include hematoma at the puncture site, dissection of the uterine artery dissection, transient sciatic nerve palsy, and synechiae, without need of surgical intervention, which are all benign complications. , , Major complications such as uterine necrosis or nontarget embolization are rare events. Endometritis and infected pelvic hematoma have been reported and usually resolve with antibiotic therapy and sometime drainage. Consequently, in women with unexplained fever after UAE, computed tomography (CT) examination should be performed to exclude abscess, pelvic thrombosis, or infected hematoma.
Uterine Atony
Uterine atony mainly occurs in women without prior obstetric history. However, multiple pregnancies, polyhydramnios or fetal macrosomia, prolonged labor, and general anesthesia are predisposing factors of uterine atony. Uterine atony represents 70% to 80% of all causes of PHH. On angiogram, uterine atony is associated with dilated uterine and arcuate arteries and there is no contrast extravasation.
Most interventional radiologists use gelatin sponge torpedoes because they are temporary occluding agents and provide proximal arterial occlusion. Nontarget embolization is the main complication, especially in the posterior trunk and external iliac artery, which can be responsible for sciatic nerve and distal leg ischemia, respectively.
The increase of arterial blood pressure after UAE is a strong indicator of clinical success. External bleeding should promptly stop and the uterus should quickly become well retracted soon after UAE. In women with persisting hypotension and hemorrhage, second-look angiography should be performed to exclude reperfusion of the bleeding site by spontaneous anastomosis. Aortogram with delayed images is needed to search for distal recanalization of uterine arteries through patent collaterals.
Placenta Accreta Spectrum Disorder
Several approaches are now available for the management of placenta accreta spectrum (PAS) disorders. They include an aggressive approach (ie, extirpative approach with placental removal, yielding high morbidity), cesarean hysterectomy (in which the placenta is removed along with the uterus, resulting in no future fertility), and full conservative management (ie, the placenta is left in place). ,
Regarding UAE, PPH caused by PAS disorder is a complex and challenging situation for several reasons. First, it is performed in an emergency setting, often with hemodynamically unstable women. Second, angiographic findings are often unpredictable, resulting in variable procedure time. Third, PAS disorder remains a main cause of failed embolization, with repeat embolization needed in a subset of patients. , ,
To date, UAE in women with PPH caused by PAS disorder is the treatment option for which the highest degrees of evidence are available. , , However, other options have been tested, including prophylactic catheter placement, balloon occlusion of the iliac arteries, and abdominal aorta balloon occlusion. ,
Uterine artery embolization
PAS disorder is a complex situation. The angiographic findings in women with PAS disorder depend on several variables. The patient may have undergone arterial ligations that result in further recruitment of extrauterine arteries. In addition, the degree of aggressiveness of PAS disorder (accreta or percreta) correlates with the technical difficulty. For these reasons, full pelvic angiogram is needed to understand the complex vascularization of the remaining placenta and pelvis. The goals of arterial embolization in women with PPH caused by PAS disorder are to stop the bleeding to avoid surgical morbidity and induce thrombosis of intervillous space, reduce the risk of further bleeding, and improve the speed of placental resorption.
The success rate of UAE in women with PPH caused by PAS disorder ranges between 76% and 92.5%. , , A systematic review including 177 women with AIP reported a success rate of 90% for UAE. Repeat embolization is feasible, resulting in an increased success rate. The need for further hysterectomy because of failed embolization is reported in 7.5% to 18% of women, with a mean rate of 11.3%. , , ,
The overall complication rate of UAE rate reaches 11%, consisting of minor complications such as pelvic pain, nausea, urticaria, and fever, or more severe complications such as deep venous thrombosis, uterine necrosis, endometritis, and synechiae. , , , , , Besides the well-acknowledged hemostatic effect of UAE, another useful role of UAE is to shorten the resorption delay of the placenta in women with PAS disorder treated conservatively with the invasive placenta left in place.
Prophylactic catheter placement and/or balloon occlusion
The use of prophylactic catheter placement and embolization in women with PAS disorder has gained recent interest because in many institutions interventional radiologists are not permanently available for emergency UAE. Several studies have investigated the potential of this approach in women with PAS disorder. The embolic agents are mainly gelatin pledgets, alone or used in combination with metallic coils. ,
Clinical success rates of up to 86% have been reported. However, hysterectomy following this approach because of ineffectiveness is needed in 8% to 30% of women. Complications include transient paresthesia of lower limb, peritonitis, and endometritis. Large blood transfusion requirement was reported in 14% of women, , with mean blood loss ranging from 1200 mL to 2279 mL and up to 9000 mL in 1 study.
Of interest, in 1 study, 2 women with prophylactic placement of catheters had severe PPH that was treated with immediate peripartum hysterectomy and UAE was not attempted, thus seriously questioning the value of prophylactic placement. In addition, 43% of women (6 out of 14) underwent hysterectomy. In addition, PPH occurred in 7 out of 14 women (50%) only, indicating that UAE may be needed in 50% of women with PAS disorder.
Balloon catheters in iliac arteries
The value of prophylactic placement of balloon catheters in the iliac arteries in women with PAS disorder is controversial, mainly owing to the higher risks of complications compared with UAE, and the lack of direct comparison with UAE. , The impact of this approach on the amount of blood loss is not fully established. One randomized controlled trial found no differences in the number of women with blood loss greater than 2500 mL, number of plasma products transfused, duration of surgery, peripartum complications, and hospitalization length using prophylactic placement of balloon catheters in the iliac arteries compared with a conventional approach. However, other studies found some benefits in terms of reduced blood loss. , In addition, only 50% of women who undergo prophylactic placement of balloon catheters in the internal iliac arteries for PAS disorder have balloon inflation during delivery when excessive bleeding occurs.
Several complications caused by the use of balloon catheters in the internal iliac arteries have been reported, with a rate of up to 16%. Balloon-related complications include left iliac artery rupture, internal iliac artery dissection, leg ischemia, and permanent claudication.
Despite using low-radiation-dose techniques, fetal radiation exposure is a major concern when considering internal iliac artery balloon occlusion. Although variable, mean fetal radiation exposure is 4.4 mGy.
Uterine Vascular Malformations
Uterine vascular malformations (UVMs) are categorized according their content and flow characteristics. , UVMs can be congenital or acquired, but congenital UVMs are less frequent than acquired UVMs. Congenital UVMs are caused by the abnormal development of a vessel resulting in multiple vascular connections invading surrounding structures. Acquired UVMs usually occur after uterine trauma such as curettage ( Fig. 5 ) or cesarean section.

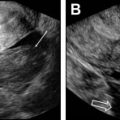
Because treatment strategy depends on the type of UVM, correct classification is essential. Slow-flow vascular malformations (venous and lymphatic malformations) are often treated by sclerotherapy, whereas fast-flow lesions (arteriovenous malformations [AVMs]) are generally managed with embolization. Fast-flow AVMs are classified and treated according to their angioarchitecture (ie, caliber and multiplicity of shuntings and draining veins). Pretherapeutic evaluation is based on transvaginal color Doppler ultrasonography and magnetic resonance (MR) imaging. Transvaginal color Doppler ultrasonography has a main role for deciding on the best treatment option. Some investigators suggest that AVMs with peak systolic velocity (PSV) less than 40 cm/s and nonsignificant bleeding should be treated expectantly, whereas those with high PSV (>80 cm/s) or significant blood flow should undergo embolization. However, the treatment should be individualized based on the clinical evaluation. MR imaging with MR angiography sequences provides useful information regarding the size and extent of AVMs. When embolization of AVMs is considered, liquid embolic agents have been reported to be useful for fast-flow AVM treatment, and seem more appropriated because they are able to progress distally through the smallest capillaries to reach the nidus, but n-butyl-2-cyanoacrylate also showed excellent results.
Benign gynecologic disease
Benign Uterine Disease
Uterine artery embolization for uterine leiomyoma
UAE is now a well-established uterine-sparing treatment of women with uterine leiomyoma. Treatment of uterine leiomyoma is restricted to symptomatic leiomyomas (ie, menorrhagia, dysmenorrhea, pain, infertility). UAE of leiomyomas allows uterus preservation and short recovery time compared with surgery. , In addition, UAE can be used in conjunction with minimally invasive surgery for the resection of multiple uterine leiomyomas. The clinical success rate for menorrhagia symptoms and bulk-related symptoms has been reported as 81% to 94% and 64% to 96% of women, respectively, along with a reduction of the uterine and leiomyoma volume of 35% to 52% and 37% to 69% respectively. Contraindications include uterine infection, pregnancy, and gynecologic malignancies. Future fertility is now considered a relative contraindication, because successful pregnancies have been reported after UAE for leiomyomas, as well as for postpartum hemorrhage and ectopic pregnancies.
MR imaging is the best imaging modality for mapping, characterization, and follow-up of uterine leiomyomas after UAE. , During the follow-up, MR imaging shows devascularization of uterine leiomyomas, which correlates with long-term success. , , The technique is similar to the general protocol of UAE in female pelvis with a few specificities: (1) microparticles 500 μm or larger are used to prevent nontargeted embolization of ovarian arteries through the utero-ovarian anastomosis ; (2) embolization should be performed distal to the cervicovaginal branch to prevent vaginal ischemia and sexual dysfunction; (3) a good result is achieved when angiogram shows a pruned-tree appearance with sluggish forward flow in the main uterine artery. Interventional radiologists should keep in mind that pseudo-occlusion caused by arterial spasm or embolic material clumping is followed by delayed restoration of flow. In addition, arterial blood supply to leiomyomas from ovarian arteries should be thoroughly investigated to prevent embolization failure. When using UAE, knowledge of the potential ovarian-uterine anastomoses is important because they provide collateral blood flow that may result in failed embolization or ovarian nontargeted embolization. Complications of arterial embolization include uterine ischemia and necrosis, unwanted embolization of cervicovaginal branches, amenorrhea, and ovarian failure.
High-intensity focused ultrasonography
High-intensity focused ultrasonography is a noninvasive, outpatient, novel treatment of uterine leiomyomas that uses tightly focused, high-energy ultrasound waves to destroy tissue, a process known as sonication. This high-energy targeted beam heats tissue to a temperature ranging between 55°C and 85°C (131°–185° Fahrenheit) resulting in coagulative necrosis and cell death. This technology can be used under MR imaging guidance (MR-guided focused ultrasonography [MRgFUS]). MR imaging provides increased image contrast and spatial resolution resulting in improved tissue targeting, and MR thermometry offers real-time thermal imaging of the treated region
Preprocedure assessment
Preprocedure contrast-enhanced MR imaging of the pelvis should be obtained to determine whether the patient is a good candidate for MRgFUS. The goal is to evaluate for size, number, location, and imaging characteristics of leiomyomas and review surrounding anatomy for treatment planning. Studies have shown that patients with fewer than 4 leiomyomas that are each less than 10 cm across or less than 500 cm 3 in volume tend to have better response. Uterine leiomyomas with homogeneous hypointense signal on T2-weighted imaging and homogeneous enhancement on fat-saturated T1-weighted postcontrast imaging have better treatment response than those with heterogeneous signal characteristics. ,
Preprocedure assessment should also include evaluation for standard contraindications to MR imaging. In addition, if the patient has an intrauterine device, it should be removed before treatment to prevent heating. The skin overlying the lower abdomen and pelvis should be evaluated for scars or tattoos along the projected beam path, which may result in skin injury or burns during the procedure.
High-intensity focused ultrasonography procedure
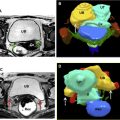
This noninvasive procedure is routinely performed in an outpatient setting with moderate sedation. The patient is positioned prone on the MR imaging table with the pelvis over the ultrasound transducer and the feet in the MR imaging bore ( Fig. 6 A). Multiplanar rapid gradient echo localizer sequences are performed to ensure appropriate positioning with the patient’s uterus in line with the transducer ( Fig. 6 B). If bowel loops are seen along the projected ultrasound beam, they can be moved by distending the bladder or filling the rectum with gel ( Fig. 7 ). Ideally the posterior aspect of the uterine leiomyoma is no more than 12 cm from the skin, and the rectum can be filled to mobilize the uterus anteriorly, if needed.


Once the patient is appropriately positioned, multiplanar T2-weighted MR imaging sequences are obtained and transferred to the workstation for treatment planning. The leiomyoma region of interest is marked and landmarks including skin, uterus, bowel, and bone are outlined to avoid injury. Multiple sonications are then performed, each lasting approximately 20 to 30 seconds, followed by a cooling period of 80 to 100 seconds. Real-time temperature measurements are also plotted throughout the sonication, and the parameters can be adjusted to ensure areas with suboptimal heating can be retreated. A typical treatment lasts approximately 3 hours. Throughout the procedure, the patient holds a panic button and can stop the sonication in case of pain or skin warmth/burning.
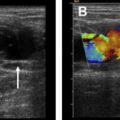
After treatment is complete, multiplanar, fat-saturated, T1-weighted noncontrast and contrast-enhanced images are obtained to evaluate procedural outcomes potential adverse events. Using the postcontrast imaging, the nonperfused volume (NPV) can be calculated. The NPV is the area of the leiomyoma that no longer enhances and does not have any blood flow ( Fig. 8 ). Technical and clinical success can be evaluated via the NPV because values greater than 50% to 60% have been correlated to improved symptoms.

Following a brief period of recovery, the patient can be discharged home. Most patients do not require any medications in the postprocedural setting. Patients are instructed to take over-the-counter acetaminophen or ibuprofen for any postprocedural pain.
Outcomes
Patients report improvement in bulk symptoms and heavy menstrual bleeding after approximately 3 menstrual cycles. Approximately 92.9% of women who undergo MRgFUS report symptom improvement at 6 months and 87.6% at 12 months. However, younger women, women with heterogeneous uterine leiomyomas on imaging, and those with NPV of less than 50% are more likely to require additional treatment. ,
A prospective randomized controlled trial that compared the efficacy of UAE versus MRgFUS in the treatment of symptomatic uterine leiomyomas found that women undergoing MRgFUS had a higher reintervention rate than those undergoing UAE. Both treatment groups experienced significant improvement in symptoms, but women undergoing UAE had greater improvement in overall quality of life and symptom scores
Adverse events
The most common side effects of the procedure are cramping and nausea/vomiting. Postprocedural pain and cramping are predominantly managed with NSAIDs, and most patients return to their normal routines after 1 to 3 days. Uncommon complications include skin burns, nerve injury, injury to adjacent organs such as bowel or ovaries, and deep venous thrombosis.
Gynecologic Infections
Pelvic inflammatory disease may be related to iatrogenic, gynecologic, or nongynecologic causes. The role of interventional radiology is to obtain a sample for diagnosis, to provide drainage, or both.
Actinomycosis is a pelvic infection that is related to long-term use of an intrauterine device. It has a characteristic feature of the infection sweeping through tissue planes and may be mistaken for malignancy. The diagnosis is often achieved by culture of the removed intrauterine device. However, sometimes biopsy of the abnormal tissue is needed. This biopsy can be performed via a transvaginal route or, if present, percutaneous biopsy of omental disease. Tuberculosis is another disease that may present as peritoneal and omental abnormal soft tissue, in which radiologically guided biopsy may be the only way to confirm the diagnosis.
Most gynecologic-related infections involve the development of a tubo-ovarian abscess. A typical scenario is someone with preexisting abnormal fallopian tubes, such as hematosalpinges, who has undergone an intrauterine intervention (coil placement, HSG, or hysteroscopy) and who then presents a few days later with pelvic sepsis ( Fig. 9 ). Management with a course of intensive antibiotics is only successful in 37% to 88% of patients. Gynecologic infections are also seen in the postoperative setting (eg, for benign/malignant gynecologic or colorectal surgery). Drainage of the resulting abscess is used to speed the recovery ( Fig. 10 ). Many of these abscesses lie deep in the pelvis and are not accessible to traditional percutaneous routes of access. Alternative routes of access are transgluteal, transvaginal, or transrectal. In general, the transrectal route should be avoided in gynecologic infections for fear of introducing bowel pathogens into the abscess. The transgluteal route has been well described. However, this can be a painful and poorly tolerated route of access, particularly if a drain is left in place.