293CHAPTER 19
SBRT for Oligometastatic Disease
Cancer Metastasis
The progression of cancer from a localized disease to metastatic disease requires a sequence of events, likely reflecting genomic and phenotypic changes. A cancer cell must first locally invade surrounding tissue and then intravasate into surrounding vasculature; it must then circulate through the body and extravasate into a similar versus different microenvironment (i.e., same vs. different organ) where it must then develop the capacity to grow at that distant site. It remains unknown whether the propensity to metastasize develops early or late in the course of cancer development, despite several genomic analyses attempting to address this question (1, 2, 3). In the late 1800s, Halsted hypothesized an orderly process wherein cancer cells spread progressively from the primary tumor into regional lymph nodes and ultimately into distant organs. In the 1980s, Fisher hypothesized an opposite extreme, on the basis of his experiments with murine breast cancer models, that cancer is metastatic from initial presentation even in the absence of clinically apparent metastatic disease (4). Recognizing that biological systems are complex and do not necessarily follow strict paradigms, Hellman proposed a model in which the extent of cancer progression in any given patient exists along a spectrum (5), ranging from a state of limited disease with the propensity to spread in an orderly, contiguous manner (as postulated by Halsted) to a state of widespread systemic disease from clinical inception (as postulated by Fisher [6, 7]). The oligometastatic state can be extrapolated from this “spectrum model” to represent an early course of metastatic progression.
The Oligometastatic State
In 1995, Hellman and Weichselbaum hypothesized a “clinical significant state of oligometastases” in which metastases limited in number and location may represent a relatively indolent disease state in which full metastatic potential is not reached (8). Resection or ablative therapy to all known metastases could lead to prolonged disease-free survival with the potential for cure in these patients (9).
Although the term “oligometastases” was coined in 1995, surgery (10, 11) and radiation (11) for limited metastases had been practiced for many decades prior to that. In 1968, Rubin questioned, Are metastases curable? in a Journal of the American Medical Association (JAMA) editorial (12) in addition to writing a book Solitary Metastasis (11) in which localized therapies were discussed. In 1983, Peters et al. explored the concept of radiation therapy as a curative treatment for metastatic disease (13), discussing the potential of systemic therapy to sterilize subclinical (i.e., occult) disease. Moreover, the importance of tumor burden of detectable disease as well as the need to deliver sufficient radiation dose while not exceeding unacceptable normal tissue tolerances was emphasized. Systemic therapy remains the standard of care for metastatic disease, and in the early to mid-20th century, less effective chemotherapy regimens and supportive care warranted attention to local therapies for metastatic disease. However, advances in systemic treatment, 294radiographic and functional imaging, minimally invasive surgical and ablative techniques, and radiation therapy have made the hypothesis of local therapy for oligometastatic disease even more compelling.
Radiation therapy for oligometastases is better suited for patients with medical comorbidities precluding surgery or invasive ablative therapies, unresectable disease, lesions in areas with significant risk for resection, and/or multiple lesions for which multiple resections would not be feasible. In the 1990s, radiation planning and delivery technology was burgeoning, with the development of three-dimensional conformal radiation therapy (3D-CRT) delivery. In fact, in their 1995 editorial, Hellman and Weichselbaum (8) noted that these technologies allow for an “increase in the tumor dose and a reduction in normal tissue toxicity by restricting as much as possible, the radiation to the accurately imaged tumor while avoiding critical normal tissues.” Over the past 20-plus years, more novel technologies have become readily available, although it should be emphasized that these technologies facilitate the treatment of oligometastases, but the concept of locally ablative treatment, often in conjunction with systemic treatment, as a potentially curable treatment option for oligometastatic disease, is independent of the technology.
Stereotactic body radiation therapy (SBRT), also called “stereotactic ablative body radiation” (SABR) therapy, has become an accepted standard in the treatment of oligometastases because of the ability to deliver high biologically effective dose, while taking advantage of a steep dose gradient (i.e., inhomogeneous dose delivery in which the maximum dose is appreciably less than the periphery of the target) such that the normal tissue volume receiving therapeutic doses is relatively low. The high fractional doses of radiation may have some biological benefits such as the ability to overcome intratumoral regional hypoxia as well as potentially stimulating an immune response (14).
SBRT FOR OLIGOMETASTATIC DISEASE
Selection Factors
Published studies generally use specific clinical criteria for the number of metastases (solitary to ≤5) to establish study eligibility, although an ideal cutoff for the number of metastases is not well established. Although a consensus definition of the “oligometastatic state” has not been reached, some have further subdivided patients on the basis of the temporal development or progression of metastases in regards to diagnosis and systemic therapy (15). De novo (also called “synchronous”) oligometastasis refer to the presence of few metastases at the time of initial diagnosis; oligorecurrence (also called “metachronous” oligometastases) refers to the development of few metastases after definitively treating the primary site, whereas “induced” or “persistent” oligometastasis describes residual metastatic lesions after response to systemic therapy. Oligoprogression describes a patient with widespread metastases with relatively stable disease on systemic therapy with only one or a few lesions demonstrating growth. SBRT (or other local therapy) is considered a means to allow continuation of the mostly effective systemic agent, with radiation addressing the oligoprogressive disease less responsive to that drug (perhaps reflecting tumor cells that have undergone a genetic change). Most of the data on outcomes after SBRT are from patients with de novo oligometastatic or oligorecurrent disease (i.e., synchronous vs. metachronous oligometastases).
Proper selection of patients who will most benefit from SBRT (or other local therapy) for oligometastases is not well understood. Unknown patient/host and tumor factors likely play a role in the propensity to develop new metastases as well as the likelihood of durable control of the treated metastases (Figure 19.1). Tumor and host biological factors are certainly important but not currently used in the clinical setting. Moffitt Cancer Center has described the use of a radiation sensitivity index (RSI) to predict for relative radiation sensitivity/resistance of oligometastases (16, 17). One major limitation of these studies, acknowledged by the authors, is that the RSI and clinical response to SBRT came from two independent datasets, precluding a direct correlation between radiosensitivity and clinical outcome. Second, it is still unclear how (or if) radiation sensitivity correlates with survival outcomes. The University of Chicago has shown that microRNAs and their target genes may regulate multiple steps in the metastatic cascade, perhaps lending itself to a better understanding of the molecular basis of oligometastatic disease (18). Among 34 patients with a variety of primary sites, metastatic sites, and histologies who underwent SBRT for oligometastases, the microRNA signature was able to differentiate oligometastatic versus polymetastatic phenotypes (19). Compellingly, microRNA expression was also able to discriminate oligo- and polymetastases phenotypes in an animal model, and investigators were able to use microRNA enhancement in an oligometastatic cell line to convert to polymetastatic progression. Similar findings were described in a study of 63 patients with resected lung metastases (20). Despite the promising early results of these bioassays, they have not yet been validated in larger clinical cohorts. Presently, practicing physicians must rely on clinicopathological factors.
295
FIGURE 19.1 Among patients with oligometastatic disease, many clinicopathological features likely affect disease control and survival as well as interact with each other.
Many studies describe patient and tumor clinicopathological factors that predict for better outcome after SBRT for oligometastases, although the question remains as to what extent of benefit (if any) local ablative therapy afforded these patients (i.e., whether better outcomes in patients with favorable prognostic factors are reflective of better response to SBRT/ablative therapy or better underlying prognosis). Nevertheless, these factors do provide insight into patient selection for SBRT. In a prospective study of 121 oligometastatic patients treated with SBRT at the University of Rochester (21), breast cancer patients fared significantly better than those with other primary sites with respect to survival and tumor control. Among those with breast cancer, one versus two to five oligometastatic sites was of borderline significance (p = .055) for survival; for non–breast cancer patients, a larger net gross tumor volume (GTV) significantly predicted for worse overall survival. Breast cancer primary site was also significantly favorable for overall survival in University of Chicago’s phase I study of 63 patients (22). In a German pooled analysis of patients with lung metastases, breast cancer primary, smaller lesion size, one versus more than one metastases, and longer duration between initial diagnosis and metastatic disease were significantly favorable factors for survival (23). In a pooled analysis of only non–small-cell lung cancer (NSCLC) patients treated with SBRT, curative-intent radiation, and/or resection, adenocarcinoma histology and metachronous (vs. synchronous) metastases were significantly favorable predictors of overall survival (24).
Many other studies (mostly single-institution and/or smaller studies) have attempted to characterize factors predictive of better survival. In a recent review by Palma et al., the “4 Aces” of major prognostic factors for patients with oligometastatic disease were described: young age (i.e., <65–70), patient fitness (i.e., Karnofsky Performance Status [KPS] ≥70), slow-growing cancers (i.e., metachronous vs. synchronous; longer duration to develop metastases), and minimal disease burden (smaller number of sites and organs; 25). On the basis of the aforementioned studies, a fifth factor (a fifth Ace or royal flush) would be favorable cancer histopathology, such as breast cancer (as discussed earlier).
Dose Fractionation
The optimal dose fractionation schedule for SBRT of oligometastases should provide a balance between tumor control probability (TCP) and normal tissue complication probability (NTCP). With increasing prescribed dose, TCP increases, eventually reaching a threshold beyond which dose-dependent incremental increases in TCP are negligible (Figure 19.2). With increased dose, NTCP risks increase. The image and/or stereotactic guidance of SBRT allows for a reduction in typical planning target volume (PTV) margins, resulting in less normal tissue dose exposure. Also, with SBRT, steep dose gradients across the target volume allow for a gradient across normal tissues, such that the volume of normal tissues exposed to therapeutic dose is minimized. Modulating the beam (i.e., intensity-modulated radiation therapy [IMRT]) to deliver a homogeneous dose to the target volume will reduce this effect, but may be preferable for complex-shaped targets, to reduce hot spots within the target and/or to reduce high-dose exposure to neighboring normal tissue. One could dose paint with IMRT in attempt to mimic a dose gradient, recognizing that the total monitor units and thus total integral dose delivered will be higher. No matter which technique is used, the normal tissue around the target will be exposed to high doses of radiation such that NTCP risks need to be considered in addition to TCP.
296
FIGURE 19.2 Treatment outcomes—biology of dose-limiting tissues.
Source: Brookhaven National Laboratory. Used with permission.
Tumor Control Probability
The ideal dose fractionation for TCP remains unknown for oligometastases and is likely dependent on many factors such as tumor bulk, histopathology, response to prior treatment, tumor location (i.e., tumor microenvironment), and unknown genotypic/phenotypic characteristics of the tumor and patient (see the introductory text of this chapter) that may dictate response to treatment. Although increasing dose seems to correlate with increased local control (26; as would be expected from classic radiobiological principles), the ideal prescription for any given patients remains difficult to ascertain, as many different fractionation schemes have been reported in published studies (typically ranging from one to 10+ fractions) and the utility of different models (i.e., linear quadratic model) to compare different fractionation schemes is not well understood and has been heavily debated (14, 27–30).
The American Society for Radiation Oncology (ASTRO) and the American College of Radiology (ACR) practice guidelines state that SBRT is used to precisely deliver one or “a small number” of fractions (31); the ASTRO and the ACR have defined SBRT earlier as the delivery of five or fewer fractions. Limiting the number of SBRT fractions to five is somewhat arbitrary, and strictly adopting such practice may hinder our understanding of the optimal dose fractionation schedules for various tumor types and treatment locations as slightly more protracted regimens may be more efficacious (in balancing NTCP and TCP) when treating larger tumors and/or tumors abutting critical organs and tissues (14).
The ongoing NRG BR002 randomized study protocol (NCT02364557, randomizing patients to standard of care with or without SBRT or surgery for one to two breast cancer oligmetastases) provides suggested one-, three-, and five-fraction prescription doses for lung (peripheral or central location), mediastinal, cervical, liver, abdominal/pelvic, spine, and bone (other than spine) sites. The protocol delineates, for each specific organ/site, both allowed and preferred number of fractions (with the “preferred” nomenclature accounting for varying practices of dose fractionation). For example, one fraction is allowed only for paraspinal/spinal sites, peripheral lung, or solitary liver metastases; three fractions are preferred for nonspinal bone, peripheral lung, liver, and abdominal/pelvic metastases; and five fractions are preferred for thoracic/cervical spine, central lung, and mediastinal/cervical lymph node metastases. The NRG BR002 also describes “per protocol” prescription doses of 30, 45, and 50 Gy for one, three, and five fractions, respectively, for nonosseous sites, and prescription doses for osseous sites of 20, 30, and 35 Gy, respectively, although a range of prescribed doses are acceptable. The NRG BR001 study is a phase I study for patients with two metastases (within 5 cm of each other) or three to four metastases to be treated with three- to five-fraction SBRT, with initial doses of 30 Gy in three fractions for spinal/paraspinal and other osseous sites, 45 Gy in three fractions for peripheral lung, liver, and abdominal/pelvic metastases, and 50 Gy in five fractions for 297central lung and mediastinal/cervical lymph node metastases; there is no plan to increase the prescribed dose; protocol doses will be de-escalated if sufficient treatment-related toxicity is observed. The ongoing randomized SABR-COMET study (NCT01446744) of SBRT for patients with one to five metastases uses single-fraction (brain and spine) and three- to eight-fraction SBRT regimens (with dose fractionation on the basis of location).
Although randomized studies will help to better define optimal practice, including the ongoing Trans-Tasman Radiation Oncology Group (TROG) of one-fraction versus four-fraction SBRT for lung metastases (NCT01965223), the number of clinical scenarios (primary site, tumor location, disease bulk) for which to study is overwhelming. In practice, it is best to adhere to published dose fractionation schedules and normal tissue constraints (see the following text) in treatment planning, with the understanding that optimal dose fractionation schemes will evolve over time as more efforts are devoted toward future investigations.
Normal Tissue Complication Probability
Several reviews have summarized published literature in an attempt to better define NTCP risks and normal tissue dose constraints after SBRT (14, 32–34). This remains a complicated area of study. Phase I studies can generally assess the tolerability within the acute phase after SBRT, but late complications can occur months to years after radiation. Furthermore, dosimetric measures extracted from dose–volume histograms do not account for organ/tissue heterogeneity or biological factors that may affect risk of radiation-related injury. Nevertheless, clinicians need guidance on dose–volume constraints (DVCs) to evaluate a plan, acknowledging that these constraints are subject to limitations.
The American Association of Physicists in Medicine (AAPM) Task Group 101 (TG-101) report (34) provides recommended DVCs for normal tissues, many of which are incorporated into the ongoing NRG BR002 randomized study. The AAPM-sponsored hypofractionated radiation therapy tumor and tissue effects in the clinic (HYTEC) reports on NTCP as well as TCP will be forthcoming in 2018, and provide some additional insight into NTCP and normal tissue dose constraints. We recommend that practitioners adhere to the AAPM TG-101 and forthcoming HYTEC guidelines. Future research efforts should be directed toward optimizing treatment parameters, such as fractional dose and total dose, as well as specific normal tissue DVCs.
PATIENT SELECTION FOR SBRT
Why SBRT?
Metastatic progression is a common occurrence in the trajectory of cancer (35). Surgical series in patients with oligometastatic disease to the lung or liver have shown that a subset of these patients will survive for a long time, with local treatments only (36–40). However, only a minority of patients will present with a limited number of metastases, and/or will be deemed suitable for surgical resection (because of comorbidities, cumulative toxicity of therapy, or anatomical location of the tumors).
In the primary and oligometastatic settings, SBRT has been reported to be an effective, minimally invasive alternative, associated with high rates of local control (70–90%) (21–23, 25, 41–47). SBRT delivers high biological equivalent doses (BEDs) to the target, while minimizing the dose to the critical structures nearby. The reported toxicity is generally low. The overall treatment time is significantly shorter than for conventional radiation therapy (1–2 weeks for SBRT vs. 6–8 weeks for conventional radiation therapy). Therefore, this technique is suitable for patients who are not candidates for other more invasive treatments. However, because of the large fractional dose, the treatment time for each fraction is longer; therefore, patient compliance is essential.
Eligibility for SBRT
Although SBRT achieves high rates of local control with minimal toxicity in patients with oligometastatic disease from different primaries, current available data failed to identify a group of patients who benefit most from these treatments. The following are recommendations for selection of patients on the basis of the currently available evidence:
• Confirmed diagnosis of malignancy
• Patients with less than five metastases in less than three organs
• Oligometastatic status confirmed with imaging—ideally PET-CT within the 2 months preceding the treatment
• Patients oligometastatic at initial diagnosis or presenting metachronous oligometastatic progression
• Unsuitable for surgical resection of metastasis/metastases
• Good performance status KPS >70% or ECOG 0–1
298Exclusion Criteria
• Widespread metastatic disease (although SBRT in the setting of oligoprogression may prove to be an exception to this recommendation)
• Short disease-free interval
• Short life expectancy (i.e., <6 months)
• Unable to achieve DVCs required because of the position or size of the lesion(s); these patients may be eligible for definitive conventionally fractionated dosing of radiation therapy
• Noncompliant patients
SBRT TECHNIQUE IN OLIGOMETASTATIC DISEASE
Although the advantage of SBRT is a steep dose gradient, allowing delivery of high biological effective dose (BED) to the target, this gradient represents a challenge with respect to setup errors, intrafraction motion, and respiratory motion of the target, any of which can lead to unwanted irradiation of normal tissues (and therefore high risk of significant unwanted toxicity) and/or underdosing of the target (and therefore risk of tumor recurrence). Concern about normal tissue exposure is especially critical for targets situated near the spinal cord, small bowel/stomach, or central lung structures. Therefore, all efforts should be made to reduce these uncertainties, while maintaining patient comfort.
Although the patients included in the published series of SBRT for oligometastatic disease comprise mostly those with a single metastasis, the definition of oligometastatic disease is inconsistent, and in some reports is considered to be up to three or five lesions, either in the same or in different organs.
Oligometastatic patients are most likely to progress with additional metastases in the same organ (48), outside the target. Some of these patients with oligorecurrences will receive further SBRT. Therefore, in patients with multiple concurrent oligometastases and in patients with oligorecurrences following previous local ablative treatments, some factors are essential when considering SBRT:
• Knowledge of each target’s motion and respiratory management
• Minimization of setup errors
• Interpretation of composite plans
• In patients with oligorecurrences treated earlier by SBRT:
• Ideally, same immobilization and same respiratory management should be used.
• Analysis of new treatment plan, taking into account previous radiation dose, especially when different dose fractionation is used, is difficult and currently empirical.
General principles of treatment planning and delivery are presented in the subsequent sections, so also the specific cases of multiple targets in the same organ, oligorecurrence, and reirradiation.
Immobilization
Comfortable immobilization of the patient reduces the risk of inter- and intrafraction errors. Patients are set up in a dorsal decubitus position, with their arms above the head. Although this position allows for more flexibility in the choice of beam directions, it can be associated with patient discomfort (because of positional pain in the shoulders), especially in the context of long treatment times (as in the case of gated or breath-hold SBRT). Patient discomfort can be associated with patient motion during treatment, therefore risk of errors in dose delivered. Several immobilization devices available are commercially available, with reported repositioning accuracy between 0.3 and 5.6 mm (34).
Body frames usually use vacuum cushions to physically immobilize the patients, while maintaining comfort. In addition, some teams use abdominal compression to reduce the motion of the targets/organs at risk (OARs) in areas with known large motion (lower lungs, upper abdomen). Although abdominal compression reduces the motion, it is often uncomfortable and has been shown to deform anatomy, depending on the positioning and the pressure applied (Figure 19.3). Some groups advocate no immobilization, favoring patient comfort. However, although current imaging systems allow for detection and correction in patient positioning, they cannot replace proper immobilization of the patients. Therefore, our recommendation is to use a vacuum-based body frame, with the patient’s arms above the head.
Special situations are the following:
• For tumors situated in the upper lobes of the lung, a head–neck–shoulder mask can be used (Figure 19.4). In this situation, the arms are situated along the body, and a customized neck support might be necessary, especially in patients with a large curvature of the cervical spine. Although the position of the arms along the body is more comfortable, the use of noncoplanar beams in these cases is usually necessary, especially for tumors near the brachial plexus.
• For tumors of the pelvis, immobilization with a vacuum cushion device may be sufficient.
299
FIGURE 19.3 Immobilization using body frame without (A) and with (B) abdominal compression. (www.qfix.com).

FIGURE 19.4 Head–neck–shoulder mask used for patients with apical lung tumors treated by SBRT.
SBRT, stereotactic body radiation therapy.
Simulation
All patients should undergo CT simulation, with a recommended CT slice thickness of 1.25 mm at the level of the tumor, 2.5 mm remaining of the scan. The recommended length of the scan is at least 10 cm above and below the target (34):
• If available, four-dimensional CT (4DCT) should be performed for treatment planning and respiratory motion analysis of the targets unless the patient is simulated with breath-hold techniques.
• If 4DCT is not available, slow acquisition CT, CT at extremes of respiration, or even fluoroscopy can be used to determine the extent of the respiratory motion of the target.
• If available, 4D PET-CT should be used, as it allows better identification of the target, and the registration-related issues are diminished. In addition to the structural information and motion information, 4D PET-CT provides metabolic data.
The advantages and disadvantages of each imaging modality are detailed in Table 19.1A.
In patients with liver metastases, fiducial markers can be helpful as the tumor is less well visualized on CT. Placement of fiducial markers is recommended to be done 7 to 10 days before the actual CT simulation is performed. As the target delineation is difficult on CT alone, MRI simulation and/or MRI registration to CT is usually required. Ideally, the MRI would be performed in the same or similar position as simulation (i.e., flat board); deformable registration of the MRI and CT will also improve target delineation.
Respiratory Motion and Its Management
Respiratory motion is an important component of the geometric uncertainty. It is patient specific and individual respiratory characteristics can vary in amplitude, period, and regularity during and between SBRT fractions (intrafraction, interfraction). In addition, it was shown that over the course of treatment, tumors may change in size, shape, and mobility. AAPM TG-76 (49) and -101 (34) recommend assessment of respiratory motion in all patients treated for moving targets, whereas the respiratory management should be individualized. Respiratory management is recommended for all tumors moving more than 5 mm in any direction.
300TABLE 19.1A Advantages and Disadvantages of Respiration-Correlated CTs
Type | Advantages | Disadvantages |
CT slow | • Ability to capture tumor motion | • Motion artifacts—blurring of the images • Loss of resolution • Errors in delineation |
CT at extreme phase of respiratory cycle | • Theoretically captures the entire range of motion • Lower workload | • Unreliable for small tumors with wide range of motion |
4DCT—gold standard | • Captures respiratory motion over few respiratory cycles • Information about the shape and mobility of tumor is acquired synchronously | • Does not take into account the daily variations in breathing pattern • Requires regular breathing pattern/ability of the patient to be coached |
Breath-hold CT | • Smallest GTV • Lung protection (DIBH) | • Patient compliance |
DIBH, deep inspiration breath-hold; GTV, gross tumor volume.
Several types of management of respiratory motion have been described in the literature. They can be grouped into two main categories:
• Passive management (motion encompassing techniques) takes into account the total range of motion and requires knowledge of the motion extent. 4DCT is typically used to determine the magnitude of motion. Although it is a valid option to manage respiration, this method is usually associated with larger volumes (especially for patients with tumors in the lower lobes of the lung, liver, etc.), and therefore a greater dose–volume radiation exposure of the neighboring normal structures (Figure 19.5).
• Active management involves either limitation of the respiratory motion (breath-hold techniques, abdominal compression) or tracking (gating; Calypso, Synchrony, ExacTrac, Vero, ViewRay, Vision/Align RT). Treatment is delivered while observing either an external or an internal marker of motion, or the actual tumor. Different correlations between external markers and internal tumor motion are reported in the literature. Although active management usually results in smaller target volumes, and therefore lesser irradiation of neighboring normal structures, it is also associated with increased treatment time; therefore, comfort and optimization of the patient before the treatment is mandatory (Figure 19.5).
Determination of Required Respiratory Management
• For targets moving less than 5 mm in any direction—free-breathing nongated treatment can be delivered. This is the most widely used technique and the easiest to implement.
• For targets presenting motion over 5 mm in any direction—the following can be implemented:
• Gating assumes a regular respiratory pattern. It minimizes the effect of respiratory motion by limiting the delivery of the treatment beam during specific portions (“gate”) of the respiratory cycle. It allows minimization of the respiratory motion and reduction of the volume of the neighboring OAR receiving unnecessary irradiation. It increases treatment time by a factor of 3. If the respiratory gate is too narrow, then it becomes impractical and another method should be used (e.g., 3D-SBRT in free-breathing or breath-hold techniques). It requires almost real-time monitoring of patient respiration.
301
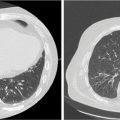
FIGURE 19.5 Example of the impact on the size of the target volume when 3D versus 4D solutions for management are used. 3D solution (encompassing the entire range of motion [A]) results in significantly larger treatment volumes compared with the volumes defined using respiratory-managed solution (B). The 3D solution will also result in larger volumes of normal structures (e.g., lung) irradiated, higher risk of radiation-induced toxicity, and possible limitation of the dose delivered. Orange contour corresponds to GTV, and the red contour to the PTV.
GTV, gross tumor volume; PTV, planning target volume.
Source: Courtesy Dwight E. Heron.
• Breath-hold is a variation of gated treatment, with the treatment beam being delivered only when the patient is holding breath in deep inspiration, deep expiration, or relaxed expiration. It is highly dependent on patient compliance and ability (i.e., his or her baseline respiratory function). It allows significant reduction of the PTV, and of the unnecessary irradiation of the OARs. However, it requires a stable baseline and the ability of the patients to hold their breath for at least 15 to 20 seconds, otherwise treatment times increase significantly. Residual motion can be present; therefore, it requires imaging-based treatment verification (i.e., fluoroscopy during breath-hold). As many patients with oligometastatic disease will eventually experience disease progression, it is suitable to reproduce the same positioning and respiratory management if subsequent radiation therapy is required. Deep inspiration breath-hold (DIBH) or end expiration breath-hold (EEBH) can be used. EEBH is reported as more reproducible and more stable; however, DIBH can be used as long as the respiratory cycle baseline (point from which the respiratory cycle starts) is stable and the patient is compliant and able to reproduce the same amplitude of inspiration (within ±3 mm).
• If a patient is unable to comply with breath-hold and exhibits an irregular respiratory cycle pattern, monophasic or biphasic coaching of the patient might be required. This is highly dependent on patient compliance and the ability of the patient to follow commands. Coaching allows regular breathing and subsequent respiratory gating; however, the amplitude of motion increases, and quality of the acquired imaging is decreased (more motion artifacts).
• For patients with irregular breathing, unable to hold their breath or follow commands, a 3D-SBRT free-breathing approach should be used .
In addition, for tumors situated near sensitive critical structures, the motion of the OARs should be analyzed and considered. For tumors in the upper abdomen, in addition to the respiratory motion, other factors to be considered are stomach fullness and peristalsis. For tumors situated in the proximity of the stomach, treatment with empty stomach is recommended (Figure 19.6).
Target Delineation
SBRT is usually used in patients with relatively small targets, for which large, ablative doses are delivered. Therefore, accurate identification and localization before and during treatment are essential. AAPM TG-101 lists several factors that impact the geometrical error, including variability in contouring of the GTV (both inter- and intraobserver) related to type of imaging used and motion artifacts (induced by both tumor and normal tissues surrounding the tumor, e.g., heart).
302
FIGURE 19.6 Example of algorithm of analysis and determination of respiratory management of target (this particular case—Varian RPM system is used for respiratory motion monitoring). (A) Passive management; (B) active management.
Although most of the groups agree that high-resolution multimodality imaging should be used for target delineation, there is no standardization of the type of imaging to be used. The majority of radiation oncologists, however, use both anatomical and metabolic imaging for target delineation. If multimodality imaging is used, we recommend dedicated scans acquired in treatment position, thin slice thickness (<3 mm), respiratory correlation, and ideally using the same respiratory management as for treatment. When multimodality imaging is used for target delineation, the accuracy of coregistration should be checked near the target, using natural landmarks or implanted fiducials (if available).
Table 19.1B presents the characteristics of the most commonly used imaging modalities for target delineation.
Although different imaging modalities can be used for target delineation, the CT planning remains the main modality used for delineation and dose calculation:
• For lung oligometastatic disease (Figure 19.7): The tumor should be contoured on the lung windows, whereas a mediastinal setting might be more useful for the OARs. Several caveats have to be taken into account.
TABLE 19.1B Imaging Modalities Used for Target Delineation
CT | • Accurate geometry, good delineation of bone, lung • Tissue density information • 4DCT available for moving targets • Need for 1.5-mm slicing or less • Windowing can impact on GTV delineation |
MRI | • Better soft-tissue contrast • T1–T2 sequences with contrast usually used • Special sequences might be required • Image spacing—1.5 mm or less (special request) • Images might have geometric distortion • Ideally scan on flat couch |
PET-CT | • Metabolic imaging not visible on CT/MRI • PET-CT images automatically coregistered • Windowing level setting may strongly affect apparent size of the tumor |
GTV, gross tumor volume.

FIGURE 19.7 CT50 and MIP CT image of lung lesion—MIP image shows extent of motion of dense lesion in lung.
MIP, maximum intensity projection.
303
FIGURE 19.8 View of GTV: smooth contouring that “makes sense in all planes.”
GTV, gross tumor volume.
• If 4D-CT scans are used, the images corresponding to the end-expiration phase (usually 50% phase) should be used for contouring.
• When maximum intensity projection (MIP) CT images are used, the delineated target will include the entire extent of motion (GTV = internal gross tumor volume [iGTV]).
• The contours should be smooth and “make sense” in all planes (Figure 19.8).
• There are guidelines regarding the inclusion of tumor spiculations seen on CT; most radiation oncologists do include only the proximal portion and not the entire extent of spiculations.
• Windowing and leveling of the planning CT scan impacts the accuracy of target delineation (e.g., window and level changes affect the apparent size of the tumor; Figure 19.9).
• For small tumors presenting significant motion, the CT imaging at extremes of expiration and extreme inspiration is not recommended for target delineation because there is a high chance that these are inaccurate in the presence of significant motion.
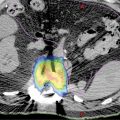
• For liver oligometastatic disease (Figure 19.5): The liver is a hypodense organ, so hypodense tumors are usually difficult to identify, even when contrast is used. For the contouring of a hypodense liver target, usually minimum intensity projection (MinIP) images should be used for contouring of the GTV (Figure 19.10). However, CT planning is not usually enough for accurate identification of the tumor, and dedicated multiphase MRI liver is recommended (Figure 19.11). The MRI sequences to be used are not standardized.
• For other abdominal targets (adrenal tumor, lymph nodes), the GTV should be delineated on a postcontrast CT scan (ideally both intravenous and oral contrast).

FIGURE 19.9 Impact of window level on the GTV delineation.
GTV, gross tumor volume.

FIGURE 19.10 MinIP images allow identification of hypo-dense liver lesion.
MinIP, minimum intensity projection.
304
FIGURE 19.11 Example of GTV contouring using CT–MRI registration. Both CT and multiphase MRI were acquired in treatment position, in end-expiration breath-hold.
GTV, gross tumor volume.
Treatment Planning
Volume Definitions
GTV: As described earlier
iGTV = GTV + margin for motion
• Adapted margins as determined during the analysis of motion are recommended unless breath-hold techniques used
• Population-based margins not recommended, as respiratory motion is patient and tumor dependent
PTV = iGTV + margins for setup errors
• Setup error is institution-specific (ability of the onboard imaging to detect tumor)—usually between 3 and 5 mm (Figure 19.12)
Organs at Risk
Radiation Therapy Oncology Group (RTOG) atlases can be used to guide the delineation of OARs. Planning organ at risk volume (PRV) = OAR + 3 to 5 mm should be used, especially when the target is situated in the immediate vicinity of an OAR (International Commission on Radiation Units and Measurements [ICRU] 52). Figure 19.13 presents definition of PRV esophagus, for a patient treated with spine stereotactic radiosurgery (SRS).
Lung Tumors (50):
• Lungs (target volume)—whole organ; although there has been inconsistency in published data on whether to subtract the GTV, iGTV, or PTV, we recommend subtracting the GTV as recommended by the RTOG

FIGURE 19.12 Algorithm for target volume definition in patients with lung oligometastases.
GTV, gross tumor volume; iGTV, internal gross tumor volume; MIP, maximum intensity projection; PTV, planning target volume.
305
FIGURE 19.13 Definition of the PRV esophagus for spine SRS.
PRV, Planning organ at risk volume; SRS, spine stereotactic radiosurgery.
• Esophagus
• Brachial plexus (for upper lobe tumors)
• Airways—major airways and second-degree ramifications
• Chest wall
• Heart—whole organ (include pericardium); delineation of cardiac substructure will likely be recommended in the near future
• Large vessels
• Skin—10 cm above and below the target (except if noncoplanar beams are used, when the length contours might be larger)
Upper Abdominal Tumors
• Liver—whole organ
• Stomach
• Duodenum
• Adjacent bowel (loops of bowel in the immediate vicinity of the target)
• Kidneys
• Other bowel (nonadjacent)
• Skin
Spine
In all patients:
• Spinal cord (should be contoured on the MRI)
• Cord PRV—spinal cord + 3 mm circumferential
• Skin
If cervical spine, add:
• Larynx
• Parotids
• Upper esophagus
• Brachial plexus
If thoracic spine, add:
• Lungs
• Esophagus
• Airways
• Heart
If low thoracic spine or lumbar spine, add:
• Adjacent bowel
• Nonadjacent bowel
• Kidneys
• Stomach
Pelvic
• Adjacent bowel
• Rectum
• Bladder
Dose Prescription and Dose–Volume Constraints
Although SBRT is increasingly used for patients with oligometastatic disease, there is significant variability in the dose fractionation, dose calculation algorithm and prescription, as well as the acceptable DVCs used. The following tables describe the most commonly used dose fractionation schedules and DVC for the most common sites of oligometastatic disease (lung, liver, adrenal, abdominal nodes, pelvic recurrences). The choice of dose fractionation is driven mainly by the position of the tumor relative to critical organs nearby, with steep dose gradients required. Documentation of the dose received, normal tissue dose exposure, and technique used (including type of respiratory management) is essential, especially if reirradiation will potentially be necessary, which is a common scenario for oligometastatic disease patients as described earlier (Table 19.2).
Lung Oligometastases
Dose fractionation schedules used for lung oligometastases mirror the ones used for treating early stage lung cancer (Table 19.3). In the literature, use of single or multiple fractions (3–4–5–8) is described, with no current standardization of 306the dose fractionation. The National Comprehensive Cancer Network (NCCN) guidelines (51) and NRG BR001 (BR001; NCT02206334) can be used as guidelines for dose fractionation schedules. Although no current standard exists, RTOG 0236 (52) clarified that fractional doses on the order of 18 to 20 Gy are unsafe for central tumors. NRG/RTOG 0813 dose escalation study will hopefully clarify the maximum tolerated dose to be used for these central and ultracentral tumors. Preliminary data reported two grade 5 cardiopulmonary toxic events in the 11.5- to 12-Gy groups (53).
307

Dose–Volume Constraints for Lung SBRT
Although there is no standardization widely acceptable for DVC for SBRT, guidelines for DVCs used for lung SBRT are currently provided by the NCCN or RTOG protocols. They are dependent on the dose fractionation schedule used (3 vs. 4 vs. 5 vs. 8). Table 19.4 provides the most commonly used DVCs for lung SBRT.

308Liver Oligometastases
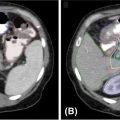
There are no randomized phase III trials in the literature comparing dose fractionation schedules for liver metastases. Retrospective and prospective phase I to II trials describe the use of 10 to 26 Gy per fraction in one to six treatments (Table 19.5) (54–59). However, there is significant variation in patient selection, histology, treated tumor volumes, and the number of treated lesions and dosimetric parameters used. Dose escalation seems to be associated with better outcomes; however, the maximum dose required to treat oligometastases is not yet defined. In addition, patients with colorectal oligometastases seem to have more radioresistant tumors and dose escalation might improve their outcomes (60). Yet, so far there is no recommendation of what dose should be used for these patients. The tumor volumes reported in the literature are generally small; however, a threshold for ineligibility for SBRT has not been yet defined. Invasion of the portal vein is rare in patients with metastatic disease; nonetheless, caution should be used when treating these patients. For metastases of the left lobe situated near the stomach and for tumors near the duodenum, the dose near these critical organs should be limited to 35 Gy per five fractions or less than 7 Gy per fraction; dose painting and treatment with empty stomach are usually necessary. In our experience, the most important parameters that drive the choice for treatment and dose fractionation remain the functional reserve of the liver (at least 700 mL liver should receive less than 15 Gy) and the position relative to sensitive structures such as duodenum/stomach.
Adrenal Oligometastases
Although adrenal metastases are common, especially in patients with NSCLC, the SBRT literature is scarce. The data come mainly from retrospective, and few prospective, studies, including usually a small number of patients, presenting adrenal metastases mainly from a NSCLC.
Similar to other oligometastatic disease sites, there is no standardization of the prescribed doses or DVC used (Table 19.5) (44, 61–67). Doses between 4 and 15 Gy per fraction have been used, in three to 10 treatments. The most commonly used dose fractionations are 24 to 45 Gy per three fractions and 40 to 45 Gy per five fractions. In a recent study from MD Anderson Cancer Center (67), no local recurrence was observed when a BED higher than 100 Gy was used. The limiting factor for dose prescribed is the dose received by the bowel, which should be limited to 35 Gy per five fractions (or less than 7 Gy per fraction). Compromise of the dose received by the PTV might be necessary to achieve the DVC. Usually, the dose is prescribed at an isodose covering 95% of the PTV; however, if compromise of the PTV coverage is required, at least 95% of the iGTV should be covered by the prescription dose.
Isolated Lymph Nodes
Few published data address the issue of SBRT in the context of isolated lymph node metastases (Table 19.6) (47, 68–73). Most of the data come from studies reporting in patients with recurrent prostate cancer. Most of the studies report the clinical target volume (CTV) defined as the macroscopic and microscopic diseases as defined on CT ± PET CT; the margins added to define the PTV are usually 1 to 1.5 cm in the craniocaudal direction and 0.5 to 0.8 cm in other directions. The majority report use of abdominal compression to reduce abdominal motion. In addition, 3-hour fasting is recommended before CT simulation and any treatment to reduce anatomical changes related to fullness of stomach and bowel.

309
Although there is variability in the dose fractionations reported, most of the studies prescribe the dose to the isodose covering 95% of the PTV. It is also agreed that if the dose constraints to OARs cannot be achieved, then the prescription dose should cover 95% of the iGTV, whereas the PTV should be covered by 80% of the prescription dose (Table 19.7).
DVC for Abdominal SBRT
Although significant variations exist in dose fractionations schedules, the majority of the teams used similar DVCs, as seen in Table 19.7.
Multiple Lesions Treated Concurrently
Although same dose fractionations and DVCs as mentioned earlier apply, the evaluation of dose conformality should be done on each individual lesion’s plan (if separate plans are used) or lesion (if one plan encompasses both lesions). When separate plans are used, the analysis of dose–volume histogram (DVH) parameters should be done on the composite plan (plan sum of individual treatment plans). This allows evaluation of the dose contribution of each plan to the OARs. In addition, if targets are situated in close proximity, analysis of composite plan indicates the dose contribution of each plan to the final dose received by each PTV. In some instances, for these closely positioned targets, creation of a single PTV might be necessary. If situated in the same organ, we recommend use of the same respiratory management for the treatment of all lesions (Figure 19.14).
TABLE 19.7 Commonly Used Dose–Volume Constraints for Abdominal SBRT
OAR | 5 Fractions | 10 Fractions |
Liver | >700 mL to receive <15 Gy | >700 mL to receive <20 Gy Mean D <20 Gy |
Stomach/small bowel | Dmax <35 Gy (any point) D0.5 mL <30 Gy V36 Gy <3% No hot spots within the stomach or bowel | Dmax <50 Gy |
Kidney | V15 Gy <35% (combined) | V15 <20% ipsilateral kidney |
Spinal cord | D0.1 mL <18 Gy | Dmax <30 Gy |
Dmax, maximum dose; OAR, organ at risk; SBRT, stereotactic body radiation therapy.
310
FIGURE 19.14 IMRT-based SBRT plan for a patient with concurrent T7 and ninth-rib oligometastases: Prescription dose for T7 was 18 Gy per three fractions to the whole vertebral body with simultaneous integrated boost delivering 24 Gy per three fractions to the GTV + 2 mm (PTV T7-SIB). The ninth-rib metastasis was prescribed 24 Gy per three fractions. For both lesions, the dose was prescribed at 98% isodose cloud. Dose conformality should be assessed on each separate plan, whereas the OARs should be assessed on the composite plan (axial, frontal, sagittal planes, as well as using the composite dose–volume histograms).
GTV, gross tumor volume; IMRT, intensity-modulated radiation therapy; OAR, organ at risk; PTV, planning target volume; SBRT, stereotactic body radiation therapy.
Treatment of Oligorecurrences Following Previous SBRT
In patients treated for oligorecurrence following previous SBRT:
• Whenever possible, use similar setup and respiratory management as for the initial SBRT.
• When available, use deformable registration (both structures and dose) to register the two different plans for further analysis. If deformable registration is not available, reproduce the previous plan and create isodose “structures” (50% of the dose prescribed earlier, 100%, or isodose levels significant for a clinical end point of an OAR, e.g., myelitis, pneumonitis).
• If the oligorecurrence is situated at a distance from the previously irradiated target, use the same dose fractionation and dose–volume constrains as given earlier.
• If the oligorecurrence is situated in the vicinity of a previously irradiated target:
• Assess eligibility for reirradiation—tolerance of the OARs and risk of toxicity should be the main criteria in the treatment choice.
• Dose fractionations and DVC should be adapted for the reirradiation situation.
• The dose received earlier by the OARs can be difficult to estimate, especially in the presence of the respiratory motion and if the respiratory management is not the same.
• There is no widely accepted model to calculate the cumulative dose received, especially in the extreme hypofractionation or when doses higher than 10 Gy are used. However, the BED remains the most commonly used model for the estimation of the doses received (74):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree