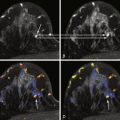
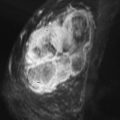
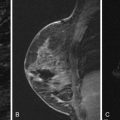
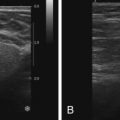
Breast cancer remains the second leading cause of cancer death in women. Mammography is the single large-scale method currently employed in screening for breast cancer in asymptomatic women. Although the effect of mammography in decreasing mortality is debated, multiple studies have shown its efficacy, particularly in patients aged 50 to 69 years. But mammographic imaging has limitations, including patient exposure to ionizing radiation and low sensitivity, especially in women with dense breasts. Between 10% and 15% of cancers may not be detected on mammography because of density of the breast tissue. Additionally, diffuse cancers and lobular carcinomas are often difficult to differentiate from normal glandular tissue on mammography. For this reason, contrast-enhanced magnetic resonance imaging (MRI), which is more sensitive than mammography, has been increasingly employed for screening purposes, in particular in populations with a relatively high lifetime risk of malignancy.
However, MRI is more logistically complex than mammography: screening MRI demands the use of intravenous contrast agent and requires exclusion of patients with magnetic resonance (MR)-incompatible hardware in their bodies. Additionally, for optimal imaging, scans should be timed for days 7 to 14 of the patient’s menstrual cycle to decrease confounding background parenchymal enhancement. Most crucially, MRI is more costly than mammography and may also lead to larger numbers of false-positive determinations and unnecessary biopsies.
In this chapter, we discuss the available literature evaluating MRI breast screening, review current guidelines for MRI breast screening, and consider controversies regarding which populations are considered appropriate candidates for screening.
Search Criteria
In evaluating the literature, we performed a search of the PubMed database using the Medical Subject Heading (MeSH) terms “early detection of cancer,” and/or “breast neoplasms/diagnosis,” and/or “breast,” and/or “magnetic resonance imaging,” and “sensitivity and specificity”; this resulted in 1564 results. After we removed duplicate entries, unrelated articles, case studies, and articles not published in English, a total of 132 articles remained.
High-Risk Groups
At present, the 2007 American Cancer Society (ACS) guidelines recommend annual MRI breast cancer screening for those with a BRCA1 or BRCA2 mutation or those with a first-degree relative with a BRCA mutation. MRI screening is also recommended for people who have a clinical history of radiation to the chest between 10 and 30 years of age and those with specific genetic mutations known to increase the risk of breast cancer such as Li-Fraumeni syndrome ( TP53 gene mutations) and Cowden and Bannayan-Riley-Ruvalcaba syndromes ( PTEN gene mutations), or their first-degree relatives ( Table 4.1 ).
| Risk | Recommendation |
|---|---|
| High risk ≥20%-25% lifetime risk of developing breast cancer BRCA1 , BRCA2 or untested first-degree relatives of BRCA mutation carriers Lifetime risk of 20%-25% or more using a breast cancer risk model History of radiation to chest between age 10 and 30 years Presence of genes known to cause Li-Fraumeni, Cowden, Bannayan-Riley-Ruvalcaba, or first-degree relatives of such carriers | Annual MRI breast cancer screening |
| Moderate risk 15%-20% lifetime risk of developing breast cancer | Insufficient evidence for or against annual MRI |
| Low risk <15% lifetime risk of developing breast cancer | No screening |
Although these known genetic mutations are associated with a lifetime risk of cancer estimated at 50% to 80%, the majority of women with a family history of breast cancer do not have an identified genetic mutation. The ACS recommends annual screening for those with a 20% to 25% or greater lifetime risk of breast cancer, a risk usually assessed by applying models that evaluate family history. Commonly used risk-prediction models include the Gail model, Claus model, and Tyrer-Cuzick model. However, a recent meta-analysis of breast cancer risk-prediction models of 18 prediction models and 7 validating studies found only consistently poor to fair discriminatory accuracy in internal and external validation. Although those models are imperfect, they remain the standard means of assessing risk for the majority of the high-risk population.
The bulk of the large prospective studies evaluating MRI sensitivity and specificity in the literature have been performed on this high-risk population ( Table 4.2 ). Table 4.3 shows that in these studies, MRI specificity (81.0% to 97.2% across studies) is consistently lower than for mammography (93.0% to 99.8%). However, MRI sensitivity (77% to 91% across studies) has consistently been shown to be greater than that of mammography (32.6% to 50.0%). As Table 4.4 shows, the yield for cancer detected on MRI alone in the compared studies ranges from 1.2% to 3.6%.
| Study | Year | Number of Patients | Risk | Mutation Carriers | Number of Patients with a Personal History of Cancer | Modalities |
|---|---|---|---|---|---|---|
| Sardanelli et al. | 2011 | 501 | ≥25% | 330 | 218 breast and/or ovary | Annual MRI, mammography, ultrasound, CBE x at least 2 rounds ∗ |
| Hagen et al. | 2007 | 491 | BRCA | All | None | Annual mammography, MRI |
| Lehman et al. | 2007 | 195 | BRCA or ≥20% chance of carrying BRCA mutation | 80 | 47 | Annual mammography, MRI, ultrasound |
| Kuhl et al. | 2005 | 529 | BRCA or suspected BRCA | 43 | 139 | Annual mammography, MRI, 6-month CBE, ultrasound |
| Leach et al. | 2005 | 649 | ≥25% | 120 | None | Annual mammography, MRI |
| Kriege et al. | 2004 | 1909 | ≥15% | 358 | None | Annual mammography, MRI, 6-month CBE |
| Warner et al. | 2004 | 236 | BRCA1 and BRCA2 | All | Breast 70 Ovary 22 | Annual mammography, MRI, ultrasound, 6-month CBE |
∗ Clinical breast examination for at least two rounds of screening.
| Study | Year | MRI: Sensitivity Specificity PPV (%) | Mammography: Sensitivity Specificity PPV (%) | Clinical Breast Exam: Sensitivity Specificity PPV (%) | Ultrasound: Sensitivity Specificity PPV (%) |
|---|---|---|---|---|---|
| Sardanelli et al. | 2011 | 91.0 96.7 56.0 | 50.0 99.0 71.4 | 17.6 99.3 56.3 | 52.0 99.0 71.4 |
| Hagen et al. | 2007 | 86.0 NR NR | 50.0 NR NR | — | — |
| Kuhl et al. | 2005 | 91.0 97.2 50.0 | 32.6 96.8 23.7 | — | 39.5 90.5 11.3 |
| Leach et al. | 2005 | 77.0 81.0 NR | 40.0 93.0 NR | — | — |
| Kriege et al. | 2004 | 79.5 89.8 60.0 ∗ | 33.3 95.0 100 ∗ | 17.9 98.1 50.0 ∗ | — |
| Warner et al. | 2004 | 77.0 95.4 42.0 † | 36.0 99.8 83.0 † | 9.1 99.3 NR | 33.0 96.0 23.0 † |
∗ PPV was analyzed per American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) categories or level of suspicion. Numbers are taken from the BI-RADS 5 or suspicious clinical breast examination category.
† PPV was analyzed per screening year. Percentages are given for year 1.
| Study | Year | Cancers | Interval Cancers | Cancers Found | Cancer Yield for MRI Alone (%) | ||
|---|---|---|---|---|---|---|---|
| Total | Invasive Breast | With MRI | With Mammography | ||||
| Sardanelli et al. | 2011 | 52 | 44 | 3 | 48 | 25 ∗ | 16/501 (3.2) |
| Hagen et al. | 2007 | 25 | 21 | 5 | 18 | 10 | 8/491 (1.6) |
| Lehman et al. | 2007 | 6 | 6 | NA (No FUP) | 6 | 2 | 4/171 (2.3) |
| Kuhl et al. | 2005 | 43 | 34 | 1 | 39 | 14 † | 19/529 (3.6) |
| Leach et al. | 2005 | 35 | 29 | 2 | 19 | 6 | 19/649 (2.9) |
| Kriege et al. | 2004 | 51 ‡ | 44 | 4 | 32 | 18 | 22/1909 (1.2) |
| Warner et al. | 2004 | 22 | 16 | 1 | 17 | 8 | 7/236 (3.0) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree