You get on the elevator. The woman in the elevator smiles at you. The door closes. She clears her throat and says, “I know you don’t remember me, but you saved my life.” She’s right—you don’t recognize her. “You read my screening mammogram five years ago—and I’m doing great. I had a small cancer, and my lymph nodes were clear. Thank you for all that you do.” You are blushing, but that just made your day!
Screening mammography is the most widely used modality for detecting breast cancer and the only one proven to reduce the mortality rate from the disease. Reading these studies, we have the chance to intervene in the natural history of a potentially fatal disease on behalf of the patient. It is a time when we can really make a difference in the life of an asymptomatic woman who feels fine. None of our other activities in breast imaging is more important.
You may recognize the names and faces of some of the women entering your mammography suite for screening. These are our colleagues, neighbors, teachers, and others who live in or near our community. They are willing to undergo the discomfort of mammography because they believe that our interpretation may provide them with the chance to have a malignancy diagnosed at a stage when cure is more likely. They are relying on us to be well trained, up to date, and free of distractions so that our attention and skills will be fully devoted to reading their mammograms.
If we are to make an impact on the breast cancer mortality rate in our local population of women, we need to be really good at interpreting screening mammograms. This assignment is unlike any other in radiology and requires different skills. We are trying to find those 3 to 8 cancers out of 1000 mammograms. Detection of those cancers can depend on perception of subtle mammographic changes that occur gradually over years. Lack of recall for a suspicious finding may be due to an error of perception (didn’t see it) or judgment (saw it and dismissed it as benign). In about 15% of cases, cancers produce no detectable signs on screening.
There are several ways to improve screening performance. Small modifications in your group practice can help optimize screening. Developing a consistent approach to reading screening mammograms reduces the chance of cancers being overlooked. Greater familiarity with the more subtle presenting signs of breast cancer will increase the cancer detection rate. Learning about common errors in judgment can push up the cancer detection rate even more. Finally, monitoring the screening performance of the overall practice and of each individual radiologist provides feedback for continual improvement.
The Prerequisites: Optimizing Your Group Practice
Stratify Screening and Diagnostic Patients
From the patient’s perspective, screening should require little time and effort. It should be convenient, quick, and performed in a pleasant environment. Providing screening facilities in the community rather than deep within the hospital increases the likelihood that women will be screened. Compliance with screening drops precipitously when the facility is more than 20 miles away, even if the test is free. Having dedicated screening facilities also allows for batch interpretation of studies. Once an abnormal finding is detected at screening, women will drive a hundred miles and even negotiate their way through your parking garage to get an answer.
Obtaining the Best Possible Images for Each Patient
A cancer can’t be detected if it is excluded from the image or cannot be seen because of blur. Thankfully, the vast majority of technologists are dedicated and passionate about our patients. They want to provide excellent care and will work hard to obtain high-quality images. Review of Chapter 1, The First Question , will provide some tools for reviewing and optimizing mammographic techniques.
When patients are recalled because of a quality issue, the technologist should review the case to make it a learning experience. A detailed audit of image quality over a period of time can provide additional specific feedback for improvement. Review quality recalls by technologist, reason (blur, positioning, other), and view. One technologist may have difficulty getting enough depth on the right craniocaudal (CC) view. Another technologist may have difficulty identifying blur (time for new glasses…). Bringing in an expert in mammographic positioning to work with your technologists every few years is a good investment.
Finally, happy technologists and staff equal happy patients. Happy patients are relaxed so they are easier to position and will also be more likely to return for annual mammography. Put staff satisfaction on the agenda for discussion at group meetings. Your staff should know that they are highly valued and that their job satisfaction is important to the radiologists and management.
Having the Right Equipment
Investing in digital mammography has had a positive impact on the quality of our practices. It has higher sensitivity and specificity than film-screen mammography for women who have dense tissue, are premenopausal, or are under age 50. Digital mammography also improves workflow and makes it easier to compare with previous mammograms.
We also recommend using computer-aided detection (CAD), which is easily integrated into the workflow using digital systems. If you take the approach of using any available information to help find cancers, the decision to use CAD is easy. Why not make use of technology that reportedly marks over 80% of screening cancers and may increase screening cancer detection by over 19%? Although the results of studies for CAD are mixed, we all have days when we are tired or distracted. CAD is especially great for marking malignant calcifications.
Digital breast tomosynthesis (DBT) is an application of digital mammography with the first commercial unit receiving Food and Drug Administration (FDA) approval in 2011. With DBT, the x-ray tube moves through an arc and multiple low-dose exposures are obtained at different angles. The data are then reconstructed into multiple tomographic images that are reviewed by scrolling through the images on a workstation. The tomosynthesis images are frequently obtained with a conventional digital mammogram (dual acquisition). This technology is intended to overcome the two major limitations of mammography: false-positive findings caused by overlapping normal and benign structures, and false-negative studies caused by obscuration of cancers by normal tissues. Tomosynthesis appears to be of greatest value in detecting and evaluating soft tissue density findings (masses, asymmetries, focal asymmetries, and architectural distortion), particularly for women with dense tissue.
The clinical use of DBT is evolving. DBT is associated with a significant reduction in recall rate of 40% to 60%. In one study, the recall rate for DBT and mammography was 4.9% compared with a rate of 11.9% for mammography alone. Between the two groups, this study found similar recall rates for masses and calcifications but a significantly lower (1.8% vs. 8.2%) recall rate for asymmetries within the DBT group. A recent prospective study of a screened population compared the performance of digital mammography alone with digital mammography and DBT. This study showed that the addition of DBT resulted in a significantly higher cancer detection rate especially of invasive cancers together with a decrease in the rate of false-positive findings. It therefore seems that DBT is particularly suited for use in the screening setting.
Who Reads the Screens?
High-volume readers generally have higher sensitivity and specificity for screening mammography. Ideally, each radiologist should read at least 1000 screening mammograms per year. In medium-size to large group practices, a subset of radiologists can be identified to batch-read screening studies. This approach is preferred over one that has more radiologists reading fewer studies. Radiologists who read screening mammograms should also see diagnostic patients and perform breast biopsy. The feedback from seeing which recalled patients are subsequently dismissed, which are recommended for biopsy, and correlating the imaging findings with pathologic findings provides the basis for continuing improvement.
Batch Versus Online Interpretation
The practice of interpreting screening mammograms in batches and recalling patients as needed for diagnostic evaluation is much more efficient than interpreting studies “online” and performing diagnostic studies during the same appointment. Batch interpretation also benefits your patients by reducing the recall rate and increasing the cancer detection rate, including the percentage of minimal and early-stage cancers. On the other hand, if your patient has limited mobility, drove hours to have her mammogram, has difficulty with transportation, or may not reliably return for recall, then an immediate reading may improve patient compliance.
Optimizing the Reading Environment
“Time to read the screens” means different things to different radiologists. For some it means it’s time for a caffeine boost. For others it’s time to put on the headphones. Whatever your personal routine, keep in mind that the mammographic signs of malignancy can be difficult to detect in even the best of conditions, and that surrounding influences in the workspace can affect the quality and efficiency of interpretation.
The optimal setting is a darkened quiet room where screening studies are reviewed in batches and reported with few interruptions. Let those emails on your smartphone wait until later. Minimize disruptive background noise. Your eyes will be at their best when background lights and computer screens are directed away from the workstation monitors and your line of sight. Computer monitors or view boxes placed away from or at a 90-degree angle to the workstation monitors minimize the effects of extraneous light.
Focus Equals Efficiency
Is your practice concerned about the efficiency of the radiologists? Whose isn’t these days! Getting into the screening mindset by batch reading and minimizing distractions focuses the radiologist on finding cancers. Getting through a worklist of screens can be very efficient with this mindset.
Learn to manage your interruptions. Interpretation of screening mammograms involves holding a visual image of a tissue pattern or questioned abnormality in mind and relating it to findings on other images as you go through the study. Let’s say that you have opened a screening study and notice a small asymmetry in the medial left breast. You keep that image in your mind while you are reviewing the rest of the study to see if it’s also on the mediolateral oblique (MLO) view and on prior mammograms. Now let’s say your technologist interrupts you in the middle and asks you to check a specimen radiograph. Do you finish the case first? Do you check the specimen and go back to where you left off? Or do you start over after you check the specimen radiograph? When you are interrupted (and you will be!), it is difficult if not impossible to remember the appearance of that asymmetry in the medial left breast. For this reason, when you are interrupted, either complete the current case first or start the whole case over after you address the issue.
Managing paperwork, preparing cases for interpretation, requesting outside studies and other patient information, and hanging and taking down analog images are functions best left to other personnel. Use of templates in voice recognition programs increases efficiency and reduces reporting errors. Standardized reporting using specific breast imaging reporting software further increases efficiency and retains data in a computerized tracking system for the medical audit.
Screening 101: Approach to Screening
Reviewing the History
Before interpreting the images, review the information provided by the technologist for history that may affect your interpretation or your callback threshold. Note any risk factors, such as a personal or family history of breast cancer. If the patient has a history of lumpectomy or benign surgery, review the location of any scars.
Occasionally, during an appointment for screening mammography, the patient will inform the technologist that she has noticed a recent change, such as a palpable finding, bloody nipple discharge, or nipple changes. The referring provider may not yet be aware of the new concern. The technologist should record this information, and it should also be described in your report. When we become aware of potentially significant new history, we schedule the patient for diagnostic evaluation.
Image Interpretation and Comparison
Establishing a systematic approach for reviewing mammograms and comparing with prior studies improves consistency and efficiency of interpretation. Current workstations for digital mammography permit great flexibility in image “hanging” protocols and sequencing of images for review. The specifics of the protocol details are a matter of personal preference; however, they should include review of all images in their entirety on high-resolution monitors, at full-pixel resolution, with enlargement or magnification.
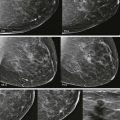
An example of a review protocol for digital mammography is shown in Figure 3-1 . Whether you use our protocol or a different one, it should be used consistently for every case. Our approach can be adapted for the interpretation of film-screen mammograms, using a handheld magnifying glass rather than digital magnification.
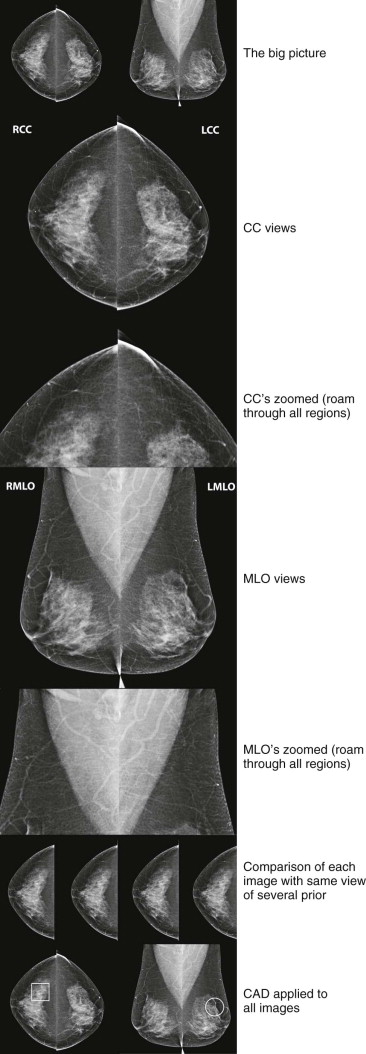
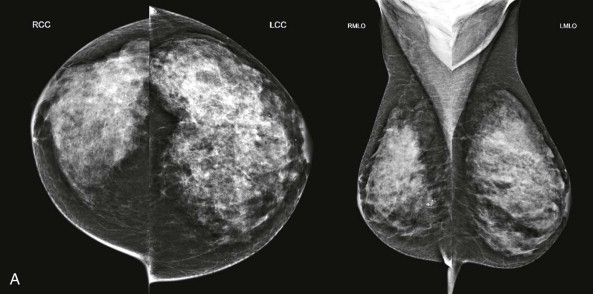
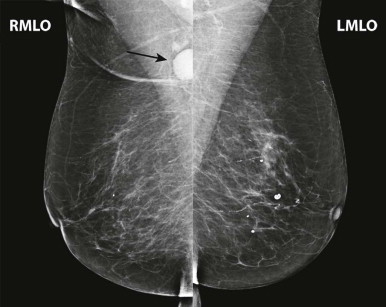
Interpretation begins with an overview of the current images in mirror-image display. In this step, look for findings that may be more obvious from a distance than close up, such as asymmetry of breast size and density, masses, suspicious tissue contours, axillary lesions, and skin or nipple abnormalities ( Figs. 3-2 and 3-3 ). More subtle findings may also be detected on the big-picture overview.

Next, carefully review zoomed images, covering all regions of the breasts on all views, searching for, yes, calcifications, but also for subtle masses, asymmetries, and architectural distortion. Closely examine any findings detected on the big-picture overview. Assess the borders of the fibroglandular tissue and examine the adjacent fat. Adjust contrast and brightness as needed to optimize evaluation of different regions.
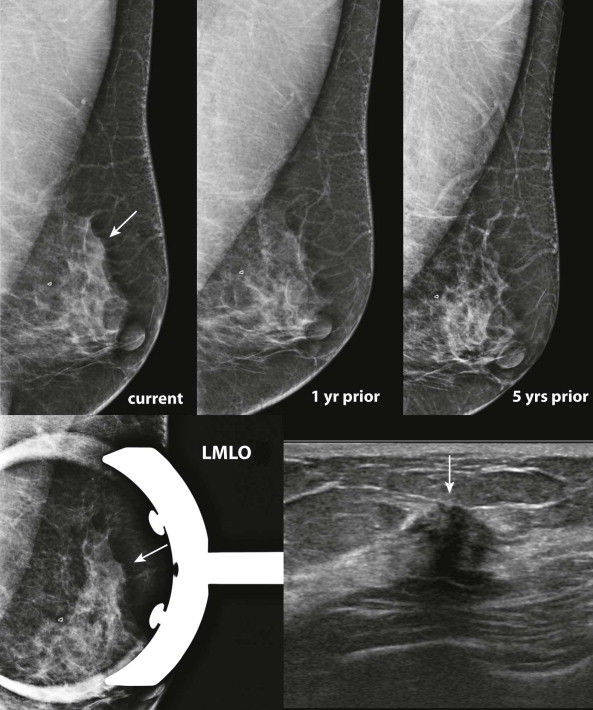
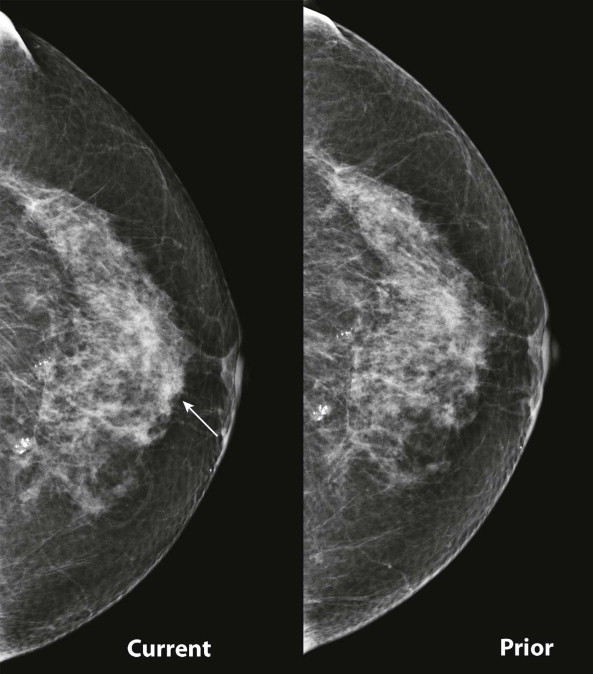
Comparison with prior studies is often needed to determine whether a finding on the current study is significant. Some findings, such as developing asymmetries or changes in the tissue contour, may resemble normal fibroglandular tissue on the current study and only be recognized as abnormal by the changes that have occurred. Stability on comparison also allows us to disregard many questioned findings.
Compare with mammograms from at least 2 years prior; even longer-term comparison may be helpful in many cases. Sometimes an apparent change from the most recent mammograms is simply due to differences in positioning that will be replicated on an earlier mammogram. In other cases, it may become more obvious that a finding is developing when comparison is made with several older mammograms. Availability of prior digital studies makes it easier to compare the current image with the corresponding previous ones in the same orientation, which can be helpful in detecting subtle changes.
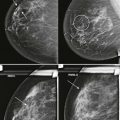
Comparing images is an active process. Identify and compare specific findings, such as masses, calcifications, asymmetries, and lymph nodes with concerning features. Also compare the tissue pattern and contours in different regions ( Fig. 3-4 ). If nipple retraction is present, see whether it is stable mammographically. Malignant findings may appear stable over years. Just because your colleague called it normal last year doesn’t mean that the finding is benign!
Finding Cancers Where They Live and Hide
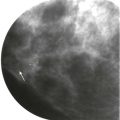
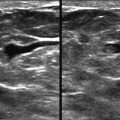
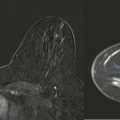
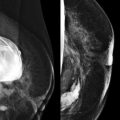
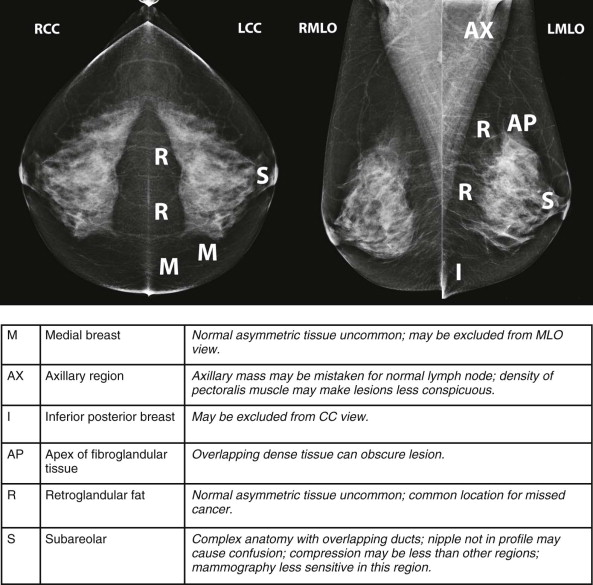
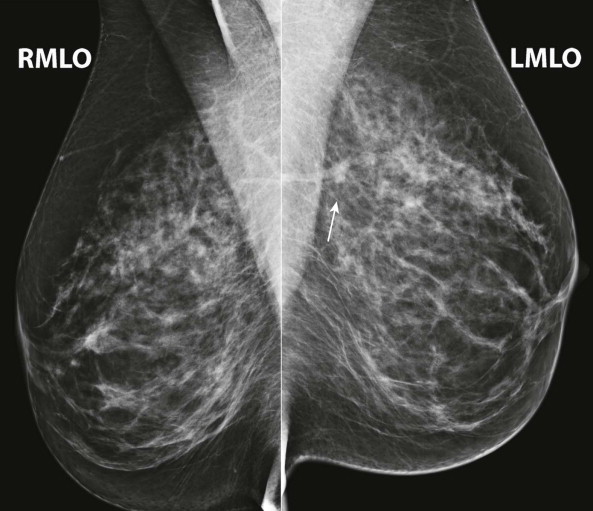
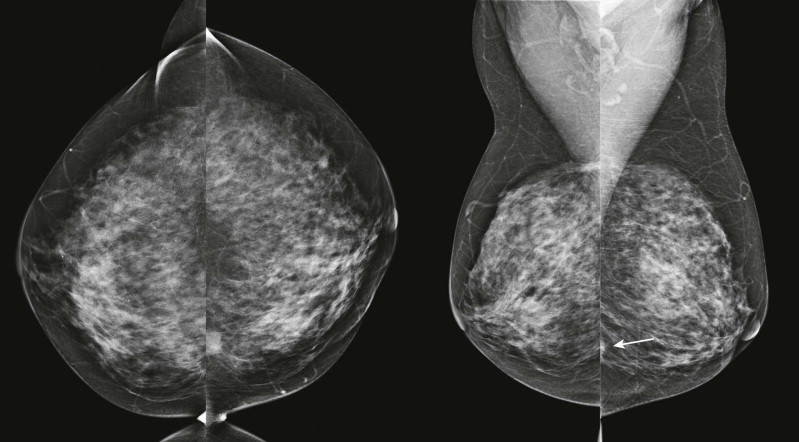
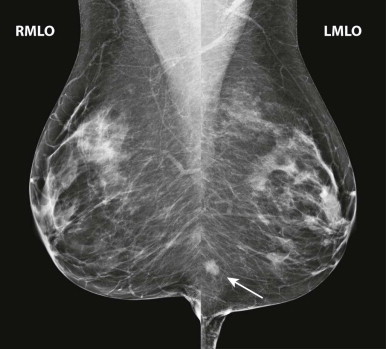
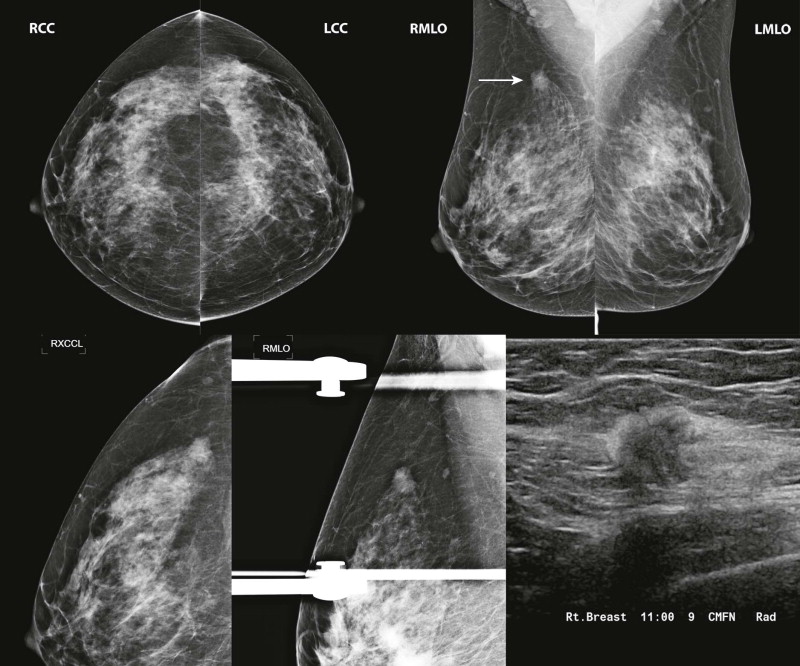
Masses and asymmetries in any region of the breast may represent malignancy. However, there are certain regions where these findings deserve your particular attention for a number of reasons. Cancers in these regions are more likely to be visible on only a single view or to be obscured by normal tissues. They may occur in areas where soft tissue density findings do not commonly develop, and should therefore be viewed with higher suspicion ( Fig. 3-5 ). These regions include the axilla (see Fig. 3-3 ), retroglandular fat ( Fig. 3-6 ), medial breast ( Fig. 3-7 ), inferior breast ( Fig. 3-8 ), apex of the fibroglandular tissue ( Fig. 3-9 ), and subareolar region ( Fig. 3-10 ).

Screening 201: Pushing Up the Cancer Detection Rate
Expand Your Breast Cancer Encyclopedia
Early in training, residents learn the classic mammographic signs of breast cancer. However, only 39% of screening-detected cancers present with classically malignant findings. As the resident sits with the attending, he or she realizes that finding early cancers requires recognition of more subtle and less specific signs, such as asymmetries and architectural distortion. (For more information on these topics, please refer to Chapters 9 and 10 .) Suddenly, the resident starts marking every little dot on the mammogram for recall. The attending smiles and says, “Cancer doesn’t look like that. That’s normal tissue.” How does the attending know what to recall and what to leave alone? Through years of experience, attendings have acquired a really big encyclopedia of the imaging appearances of breast cancer.
Familiarity with the varied appearances of malignancy is essential to increasing cancer detection on mammography. Finding breast cancer is like looking for a familiar face in a crowd. Our eyes quickly scan over the crowd with an image of the person in mind. When our eyes cast over the familiar face, recognition is usually instantaneous. We don’t search consciously for individual features, such as facial bone structure or hair color; we recognize the person who embodies a composite of these features. The more people we know in the crowd, the more faces we will recognize.
There is evidence that the same is true in detecting the many faces of cancer on screening mammograms. Kundel and colleagues evaluated search time and gaze tracking during analysis of a series of mammograms, half with subtle malignant findings. They found evidence for a very rapid holistic process for detecting and analyzing subtle signs of malignancy that was most highly developed among the more proficient observers. Less experienced observers relied more heavily on a search-to-find approach, which was slower and associated with more errors. These observers were less likely to detect malignant findings on rapid holistic review and also less likely to recognize the significance of the malignant findings that were detected. As we become more familiar with the subtle presentations of malignancy, our eyes will be increasingly drawn to them.
How do we gain experience recognizing these subtle findings? Look at every breast cancer that you can! Every time that you read a mammogram on a patient who has had breast cancer, pull up the mammograms from the time of her diagnosis. And then, if you really want to see what early breast cancer looks like, pull up the mammograms from a year or 2 before that. It is common to see some evidence of the cancer on preceding mammograms. It doesn’t mean that your colleagues made a mistake, but it will help you burn those faces of cancer into your brain. Use of educational materials that demonstrate numerous case images of malignancies will also accelerate the learning process. Adding these images to your repertoire of the signs of malignant lesions will make it easier to recognize similar findings as they are encountered prospectively.
It is also important to become very familiar with the mammographic appearance of findings that do not represent cancer. Performing diagnostic mammography in addition to screening builds experience with the varied appearance of normal tissue patterns and will also aid in the recognition of findings where those patterns are altered. Practices where radiologists read only screens are at a disadvantage because they do not get the benefit of working up findings and learning which are normal and which require biopsy.
Balancing the Decision to Recall
Reading screening mammograms is all about the odds. How likely is a finding to represent cancer? Radiologists understand that over 99% of screening mammograms will not reveal cancer. How do we know if we’re calling back too few or too many patients?
In the past, the recommended callback rate was a number specifying an upper limit of acceptability, usually 10%. In 2010, Carney and associates defined an acceptable callback range of 5% to 12%. Establishing a lower limit acknowledges that recalling fewer than 5% of patients makes it very difficult to detect and diagnose many of the more subtle malignancies that present with less specific findings. The emphasis is usually placed on how to lower the recall rate, and that is important. However, a low recall rate is unimportant if the cancer detection rate is low. Both recall rate and cancer detection rate should be looked at together to assess performance.
The highest priority in screening is to establish a high cancer detection rate. Recently trained radiologists often need support early in practice to gain experience and develop effective practice habits. Early on, these radiologists tend take longer to interpret cases and recall a higher percentage of patients than radiologists with more experience. This is to be expected, and usually, as experience is gained, efficiency increases and the callback rate decreases. Findings to consider for recall are summarized in Box 3-1 .