The various findings observed on computed tomography (CT) and magnetic resonance (MR) imaging examinations in patients with seizures reflect the variety of different causes that give rise to this common neurologic symptom. In the emergency setting, CT is most valuable in its ability to accurately identify acute abnormalities that require emergent medical or surgical treatment. MR imaging, by contrast, is usually reserved for patients with recurrent or refractory seizures. The accurate interpretation of either modality requires familiarity with how seizures are classified clinically, the most common presenting features of different causes for seizures, the relevant neuroanatomy, and the imaging manifestations of both common and uncommon causes of seizures and epilepsy. Of particular practical importance to the radiologist is the ability to recognize (1) the most common findings in patients with recurrent seizures and (2) potentially reversible causes for seizures that require prompt intervention to avoid or minimize permanent brain injury. This article surveys a variety of different causes for seizures and epilepsy, focusing on specific clinical features that can help to refine differential diagnosis, and on imaging findings characteristic of different disorders.
The term seizure refers to an abrupt but transient interruption in normal brain function that comes about as the result of an unregulated discharge of neurons. Seizures are so common that nearly 10% of the population of the United States will have at least one seizure by age 80. New unprovoked seizures or seizures that are not rapidly controlled are viewed as a neurologic emergency, and thus in the emergent setting often undergo computed tomography (CT) scanning or less commonly, magnetic resonance (MR) imaging. Epilepsy, in contrast, is a term reserved for the chronic brain disorder characterized by recurrent and unpredictable seizures without evidence for a reversible systemic cause. The prevalence of this disorder in the United States is estimated at 0.5% to 1%. For the evaluation of patients with epilepsy, MR imaging is almost always the first-line imaging modality. Positron emission tomography (PET) with 18 F fluorodeoxyglucose (FDG) and magnetoencephalography (MEG) may also be used in some centers to increase diagnostic sensitivity and specificity in cases where high-quality MR imaging is negative or equivocal.
This article focuses on CT and MR imaging of unusual and potentially reversible causes for acute seizures in the emergency setting. Because many patients who develop seizures after trauma, infection, or metabolic derangements may eventually develop epilepsy, some of the chronic brain conditions that result secondarily from an acute event are also included in this review. In particular, posttraumatic epilepsy and mesial temporal sclerosis (MTS) are discussed in some detail. The interested reader is referred to several excellent articles and texts for a more comprehensive review of imaging in epilepsy, especially with regard to malformations of cortical development, phakomatoses, and emerging advanced MR imaging, MEG, and nuclear medicine techniques for improving localization of seizure substrates when MR imaging is unrevealing.
Seizure types and indications for imaging
As a single symptom of a broad range of neurologic disorders ranging from Alzheimer to Zellweger disease, seizures have a variety of imaging manifestations. Some of these are common and some are exceedingly rare. Many findings that have been observed represent the secondary hemodynamic, metabolic, or excitotoxic effects of seizures on the brain. Others such as MTS, infarcts, tumors, or cortical dysplasia can represent the primary underlying cause of seizures. When imaging patients presenting with new or recurrent seizures, it is important that the radiologist be able to confidently distinguish between potential causes and the secondary effects of seizure activity, especially with regard to recognizing causes that may be reversible with the institution of appropriate treatment. In the emergency setting, imaging is commonly performed with CT. When CT is contraindicated or unhelpful, MR imaging may also reveal reversible causes for seizures, or may disclose findings that suggest the next step in the workup of seizures.
As detailed in the recently revised terminology for classification of epilepsy developed by the International League Against Epilepsy (ILAE), 2 principal seizure types are recognized: focal seizures (or partial seizures), which show clinical or electroencephalographic (EEG) evidence for origin from a discrete location within the cortical or subcortical gray matter of one hemisphere, and generalized seizures, which may also arise from a single discrete location but in contrast to focal seizures rapidly propagate through both hemispheres and thus clinically do not have consistently localizing features. (Modern imaging, methods for genomic analysis, and molecular biology have significantly altered our understanding of seizures since the initial ILAE classification was published in 1960, and updated for seizures in 1981 and for epilepsy in 1989. The latest proposed revision of the ILAE classification can be found in Ref. ) This distinction is important, as the causes, treatment, and outcomes for these 2 types of seizures differ significantly and vary by age. Chronic generalized seizures often present in childhood and usually have normal imaging; acute generalized seizures in the adult are more ominous, as they are more likely to have significant structural lesions evident on routine CT. In contrast, both acute and chronic partial seizures are more likely to have imaging abnormalities that are occult on CT and sometimes exceedingly subtle on MR imaging. In this regard, it is important to recognize the limitations of CT. Adults with acute generalized seizures should be evaluated clinically for evidence of trauma, metabolic abnormalities, or toxic ingestion, with a low threshold for CT imaging when a significant intracranial process such as infection, tumor, or hemorrhage is suspected. In some cases, however, the evaluation should not stop when CT does not disclose any significant abnormality, but rather should include additional imaging with MR.
Multidisciplinary guidelines developed through the process of expert review have led to the development of well-defined criteria for determining whether CT and/or MR imaging are indicated in the emergency setting. As a general rule, imaging is recommended for any patient in whom a serious structural lesion is suspected based on history and physical examination, or when a clear systemic cause for seizures has not been identified . Recommendations differ based on age and clinical presentation, with imaging strongly suggested after first-time seizures in patients older than 40, in cases of known malignancy or immune compromise, or when symptoms suggest a partial onset (all conditions that increase the likelihood of a focal brain lesion). Even in patients known to have epilepsy, repeat imaging is recommended when the seizure pattern or type changes, when seizures result in significant head trauma, or when prolonged postictal confusion or declining mental status are present. Appropriateness criteria issued by the American College of Radiology also serve as a useful resource to aid in the selection of patients for imaging.
Patients with acute intracranial hemorrhage, brain tumors, infections, dural venous sinus thrombosis, traumatic brain injury (TBI), developmental abnormalities, and vascular lesions such as cavernous and arteriovenous malformations may all present with seizures. These entities are discussed in other articles in this issue.
In a cohort of 880 individuals from the city of Rochester, Minnesota, no specific cause for seizures was found in 65.5% of patients ; however, disorders with abnormalities that could potentially be identified by imaging were also common in this cohort. These abnormalities included cerebrovascular disease in 10.9%, congenital lesions in 8.0%, trauma in 5.5%, tumors in 4.1%, neurodegenerative disease such as Alzheimer disease in 3.5%, and infection in 2.5%. The proportion of cases with underlying brain structural abnormalities varied by age, with tumors and infarcts far more common among patients older than 65 than among those in younger age groups, where infection and trauma were more frequent. Metabolic derangements and alcohol withdrawal collectively represented common causes for seizures among the middle-aged population. In children between the ages of approximately 5 months and 6 years, many series have shown that most seizures represent simple febrile seizures. Imaging in this patient population is usually unwarranted at first presentation, especially in light of growing concerns regarding radiation exposure and the availability of established clinical practice guidelines that do not routinely include CT.
Logistic and cost issues aside the diagnostic yield of MR imaging is far higher than that of CT in both patients with acute seizures and patients with long-standing epilepsy. Not all MR imaging examinations are equivalent, however. The accuracy of MR imaging increases with the field strength used for acquisition, as the higher signal-to-noise and contrast-to-noise afforded by 3-T imaging allow visualization of smaller structures and more small variations in signal intensity than conventional 1.5-T imaging. MR imaging studies performed for the evaluation of seizures should also be tailored to optimize detection of seizure foci, as routine brain protocols may miss lesions responsible for seizures. Finally, although there is evidence that general radiologists consistently detect critical structural abnormalities, subtle abnormalities may be detected at higher rates by subspecialty-trained neuroradiologists practicing in dedicated epilepsy centers. According to one study, for example, the diagnosis of hippocampal sclerosis was missed prospectively in 86% of initial imaging reports. Although controversial, this study at the very least reiterates the need for all radiologists to: (1) increase their familiarity with the MR imaging diagnosis of MTS (discussed later in this article), (2) employ dedicated high-resolution seizure protocols, and (3) incorporate clinical and EEG information regarding seizure type and localization into the process of interpretation.
Modern seizure evaluation should routinely include coronal thin-section T2-weighted images of the medial temporal lobes and a volumetric T1-weighted evaluation of the entire brain to allow critical assessment of regional sulcal anatomy, cortical thickness, and definition of gray-white boundaries. In addition, it is important to include sequences sensitive to magnetic susceptibility to identify calcification and chronic blood products from prior trauma, cavernous malformations, and infections such as neurocysticercosis (one of the most important causes for seizures worldwide). New three-dimensional (3D) phase-sensitive susceptibility-weighted techniques likely have higher sensitivity in this regard than conventional gradient echo T2* sequences. Of note, high spatial resolution, formerly possible only with specialized surface coils, can now be routinely obtained with most sequences on 3-T scanners using phased-array receive coils. Gadolinium-enhanced images are not typically required for evaluation of chronic seizures unless a tumor is suspected. In the acute setting, however, contrast-enhanced sequences may be useful to evaluate for underlying infections, tumors, or vascular lesions ( Table 1 ).
| Sequence | TR/TE/TI (ms) | FOV (mm) | Matrix | Slice/Gap (mm) | |
|---|---|---|---|---|---|
| 1 | Axial DWI (b = 1000 s/mm 2 ) | 10000/MIN | 220 × 220 | 128 × 128 | 3 skip 0 |
| 2 | Coronal T2 FSE-IR | 5000/120 (ETL 16) | 220 × 160 | 512 × 256 | 3 skip 0 |
| 3 | Coronal 3D T1 a | 36/MIN | 220 × 160 | 230 × 230 | 1.2 skip 0 |
| 4 | Coronal T2 FLAIR b | 10000/140/2200 | 220 × 160 | 256 × 192 | 3 skip 0 |
| 5 | Axial T2 FSE | 5850/100 (ETL 17) | 220 × 220 | 384 × 384 | 3 skip 0 |
| 6 | Coronal T2* GRE c | 787/25 | 220 × 160 | 256 × 192 | 5 skip 1 |
| 7 | Gad axial T1 SE | 600/MIN | 220 × 220 | 256 × 192 | 5 skip 1 |
| 8 | Gad coronal T1 SE | 600/MIN | 220 × 160 | 256 × 192 | 5 skip 1 |
a Volumetric spoiled gradient echo (SPGR) or magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequences are recommended to optimize gray-white contrast with high spatial resolution. Images should be reformatted and reviewed in 3 planes.
b Volumetric FLAIR sequences are also useful when available, as they facilitate review in 3 planes at high spatial resolution.
c 3D susceptibility-sensitive sequences may be substituted for conventional T2* gradient echo imaging.
Peri-ictal changes on imaging
Independent of cause, seizures are associated with dramatic alterations in cellular metabolism, disruptions in normal cerebral autoregulation, shifts in relative compartmental water distributions, and changes in intracellular ion concentrations. The variety of pathophysiological events that take place as the direct result of seizure activity is reflected in the myriad imaging findings that have been described with ongoing or recent seizure activity. These “peri-ictal” imaging abnormalities may be reversible or irreversible, depending on the nature and duration of seizures and, like the underlying electrochemical events that cause them, may affect the brain in a focal or diffuse fashion.
Often mild or absent on unenhanced CT, peri-ictal changes commonly appear as patchy areas of high signal on T2, fluid-attenuated inversion recovery (FLAIR), or diffusion-weighted MR imaging ( Fig. 1 ). Typically involving gray matter, either within the cerebral cortex or in the deep gray nuclei, these abnormalities are frequently bilateral and may be migratory on serial imaging. Peri-ictal changes may arise within a localized epileptogenic region of the brain or remotely within areas of the brain distant from the actual seizure focus. Changes in the region of seizure onset are thought to be the direct result of localized metabolic and vascular changes associated with abnormal neuronal discharge ; the precise cause for remote changes is more difficult to understand. Frequent involvement of limbic structures, especially the hippocampus, suggests that seizures preferentially involve neuronal populations with a lower intrinsic seizure threshold. Alternatively, as suggested by signal changes in the thalamus or cerebellum, seizures may propagate secondarily through distributed networks in the brain.
The signal abnormalities related to seizures on MR imaging are commonly associated with mild mass effect, causing subtle sulcal effacement within involved areas. Occasionally, seizures may be associated with more pronounced mass effect. In these cases, imaging may erroneously lead to the diagnosis of a mass lesion or, in patients with known tumors, be mistaken for tumor progression. The transient and migratory nature of these changes on serial imaging and associated clinical picture may provide the only clues that the abnormalities are related to seizures. Other reported, but less common, findings include transient sulcal hyperintensity on FLAIR sequences, leptomeningeal enhancement, cross-cerebellar diaschisis, and asymmetric enlargement of arterial branches on MR angiography. These less common findings may confound the diagnosis of postictal changes; follow-up imaging can be useful in assessing the degree to which abnormalities are reversible and in differentiating postictal changes from other disorders that may have similar imaging features, such as encephalitis and infarction. Short-term follow-up imaging and/or lumbar puncture may be warranted to differentiate among these different possibilities.
The observation of peri-ictal changes on MR imaging depends on the duration of seizures and the time between the cessation of seizure activity and imaging. Transient partial seizures are infrequently associated with peri-ictal changes whereas prolonged, refractory seizures or status epilepticus are more likely to exhibit these abnormalities. The exact timing and duration of seizure activity is often difficult to establish with certainty because whereas generalized tonic-clonic seizures are dramatic and more readily documented, partial or nonconvulsive seizures may be clinically occult. In the authors’ experience, patients with underlying comorbid diseases such as lupus, immunocompromised patients, solid organ transplant patients, and patients on chemotherapy agents are also more likely to show peri-ictal changes on MR imaging.
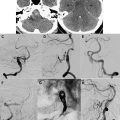
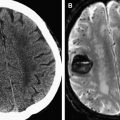
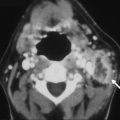
Peri-ictal changes may be transient and reversible, or they may progress to permanent cell death and gliosis. The extent of brain injury may be evident clinically. However, longitudinal follow-up in some cases after prolonged seizures can provide supplementary evidence for permanent brain injury, usually manifest as focal or global cerebral atrophy or cortical injury, including laminar necrosis on T1-weighted images ( Fig. 2 ). Cytotoxic edema, as implied by the presence of reduced diffusion, is a more ominous finding, but does not necessarily imply irreversible injury. One interesting location for high T2 signal and reduced diffusion that is typically reversible is the splenium of the corpus callosum ( Fig. 3 ), in which a rare circumscribed lesion has been variably ascribed to certain antiepileptic medications such as vigabatrin or to seizures themselves. The reversibility of this finding suggests that reduced diffusion in this lesion does not reflect cytotoxic edema; a transient disturbance in energy metabolism and ionic transport that results in reversible myelin vacuolization or intramyelinic edema has been proposed.
Peri-ictal changes on imaging
Independent of cause, seizures are associated with dramatic alterations in cellular metabolism, disruptions in normal cerebral autoregulation, shifts in relative compartmental water distributions, and changes in intracellular ion concentrations. The variety of pathophysiological events that take place as the direct result of seizure activity is reflected in the myriad imaging findings that have been described with ongoing or recent seizure activity. These “peri-ictal” imaging abnormalities may be reversible or irreversible, depending on the nature and duration of seizures and, like the underlying electrochemical events that cause them, may affect the brain in a focal or diffuse fashion.
Often mild or absent on unenhanced CT, peri-ictal changes commonly appear as patchy areas of high signal on T2, fluid-attenuated inversion recovery (FLAIR), or diffusion-weighted MR imaging ( Fig. 1 ). Typically involving gray matter, either within the cerebral cortex or in the deep gray nuclei, these abnormalities are frequently bilateral and may be migratory on serial imaging. Peri-ictal changes may arise within a localized epileptogenic region of the brain or remotely within areas of the brain distant from the actual seizure focus. Changes in the region of seizure onset are thought to be the direct result of localized metabolic and vascular changes associated with abnormal neuronal discharge ; the precise cause for remote changes is more difficult to understand. Frequent involvement of limbic structures, especially the hippocampus, suggests that seizures preferentially involve neuronal populations with a lower intrinsic seizure threshold. Alternatively, as suggested by signal changes in the thalamus or cerebellum, seizures may propagate secondarily through distributed networks in the brain.
The signal abnormalities related to seizures on MR imaging are commonly associated with mild mass effect, causing subtle sulcal effacement within involved areas. Occasionally, seizures may be associated with more pronounced mass effect. In these cases, imaging may erroneously lead to the diagnosis of a mass lesion or, in patients with known tumors, be mistaken for tumor progression. The transient and migratory nature of these changes on serial imaging and associated clinical picture may provide the only clues that the abnormalities are related to seizures. Other reported, but less common, findings include transient sulcal hyperintensity on FLAIR sequences, leptomeningeal enhancement, cross-cerebellar diaschisis, and asymmetric enlargement of arterial branches on MR angiography. These less common findings may confound the diagnosis of postictal changes; follow-up imaging can be useful in assessing the degree to which abnormalities are reversible and in differentiating postictal changes from other disorders that may have similar imaging features, such as encephalitis and infarction. Short-term follow-up imaging and/or lumbar puncture may be warranted to differentiate among these different possibilities.
The observation of peri-ictal changes on MR imaging depends on the duration of seizures and the time between the cessation of seizure activity and imaging. Transient partial seizures are infrequently associated with peri-ictal changes whereas prolonged, refractory seizures or status epilepticus are more likely to exhibit these abnormalities. The exact timing and duration of seizure activity is often difficult to establish with certainty because whereas generalized tonic-clonic seizures are dramatic and more readily documented, partial or nonconvulsive seizures may be clinically occult. In the authors’ experience, patients with underlying comorbid diseases such as lupus, immunocompromised patients, solid organ transplant patients, and patients on chemotherapy agents are also more likely to show peri-ictal changes on MR imaging.
Peri-ictal changes may be transient and reversible, or they may progress to permanent cell death and gliosis. The extent of brain injury may be evident clinically. However, longitudinal follow-up in some cases after prolonged seizures can provide supplementary evidence for permanent brain injury, usually manifest as focal or global cerebral atrophy or cortical injury, including laminar necrosis on T1-weighted images ( Fig. 2 ). Cytotoxic edema, as implied by the presence of reduced diffusion, is a more ominous finding, but does not necessarily imply irreversible injury. One interesting location for high T2 signal and reduced diffusion that is typically reversible is the splenium of the corpus callosum ( Fig. 3 ), in which a rare circumscribed lesion has been variably ascribed to certain antiepileptic medications such as vigabatrin or to seizures themselves. The reversibility of this finding suggests that reduced diffusion in this lesion does not reflect cytotoxic edema; a transient disturbance in energy metabolism and ionic transport that results in reversible myelin vacuolization or intramyelinic edema has been proposed.
Posterior reversible encephalopathy syndrome
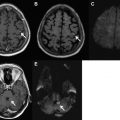
Peri-ictal changes may overlap with the so-called posterior reversible encephalopathy syndrome (PRES), a controversial entity that may also be associated with seizures. Several conditions have been associated with this disorder, including hypertension, eclampsia, certain medications, infection, autoimmune diseases, hypercalcemia, hemolytic uremic syndrome, and renal failure. Seizures in PRES clinically may initially show focal onset, though frequently generalize and often recur. The pathogenesis of PRES is poorly understood and controversial. One popular hypothesis is that the disorder results from loss of normal cerebral autoregulation with consequent hyperperfusion. Another suggests vasoconstriction with hypoperfusion, and yet another implicating endothelial cell dysfunction.
Following the brain watershed zones, signal abnormalities in cases in PRES are most often bilateral and symmetric. As implied by the acronym, typical findings are reversible focal or patchy areas of T2/FLAIR hyperintensity in the subcortical white matter, especially within the parietal and occipital lobes. The imaging manifestations are broader than the moniker implies, however, and include irreversible cytotoxic injury, abnormalities in both gray and white matter, and involvement of not only the parietal and occipital lobes but also the frontal lobes, the inferior temporo-occipital junction, thalami and basal ganglia, brainstem, and cerebellum. Cross-sectional and catheter angiographic studies may show vasospasm, vasodilation, and/or beading. Hemorrhage occurs in 15% to 20% of cases. These less common imaging findings may be mistaken for other disorders such as infarct or tumor, especially when reduced diffusion, marked asymmetry, mass effect, and/or enhancement is present ( Fig. 4 ).