A 62-Year-Old Male With a 6-Month History of Progressive Dyspnea
- Claudia Patricia Jaimes Castellanos, MD
Synopsis
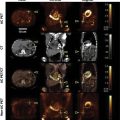
A 62-year-old male presented with a 6-month history of progressive dyspnea, worsening NYHA class II to class IV, orthopnea, and lower extremity edema. His medical history was significant for hypertension. On physical exam, vital signs were normal (BP: 100/60, HR: 88 bpm, O2Sat: 92%), and demonstrated elevated jugular venous pressure, abdominal distension, and mild lower extremity edema. Chest X-ray showed a normal cardiac silhouette, right pleural effusion, thickening of the minor fissure, and interstitial markings ( Fig. 16.1.1 ). ECG: sinus rhythm, with nonspecific ST segment abnormalities. A transthoracic echocardiography was performed. Findings ( Figs. 16.1.2–16.1.3 ) were suggestive of pericardial constriction. The patient was referred for cardiac magnetic resonance (CMR). Findings ( Figs. 16.1.4–16.1.7 ) were consistent with the diagnosis of constrictive pericarditis and suggested active inflammation. A summary of the noninvasive imaging findings is provided in Table 16.1.1 .







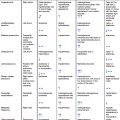
| Modality | Summary of Findings | Figure No. |
|---|---|---|
| CXR | AP and lateral films show normal cardiothoracic index and clean lung fields. No flow redistribution is observed. Within the cardiac area, hyperintensity is compatible with calcifications within the cardiac silhouette, normal cardiac silhouette, right pleural effusion, thickening of the minor fissure, and interstitial markings. | Fig. 16.1.1 |
| TTE | Normal size left ventricle, with septal bounce and diffuse hypokinesia, with moderate systolic dysfunction, LVEF: 36%, and dilated left atrium. M-mode demonstrates septal bounce. | Fig. 16.1.2A |
| Mitral inflow Doppler shows a restrictive filling pattern. | Fig. 16.1.2B | |
| Respiratory variation of mitral and tricuspid flow. | Fig. 16.1.2C | |
| Mitral annular tissue Doppler is consistent with annulus reversus, medial tissue annular velocity > 8 cm/s. | Fig. 16.1.2D–E | |
| Hepatic vein Doppler shows reverse diastolic flow during expiration. | Fig. 16.1.2F | |
| Subcostal view shows (A) pericardial thickening and (B) IVC plethora. | Fig. 16.1.3A–B | |
| CMR | SSFP Cine sequences in diastole (A) and systole (B) show normal size of the left and right ventricles, with mild LV systolic dysfunction (EF = 48%), and marked thickening of the pericardium | Fig. 16.1.4 |
| Real-time sequence showed normal appearance of the interventricular septum during expiration (A) and flattening during inspiration (B) | Fig. 16.1.5 | |
| T1-W sequence showing pericardial thickening (top); T2W sequences with fat saturation, showing pericardial edema (bottom). | Fig. 16.1.6 | |
| PSIR 4-chamber view shows diffuse pericardial LGE. | Fig. 16.1.7 |
Pericardial biopsy showed chronic pericardial fibrosis, severe inflammation, caseating granulomas, and the presence of acid-fast bacilli (AFB) on Ziehl-Neelsen-stained smears compatible with tuberculous constrictive pericarditis. He received anti-TB treatment for 6 months and clinical improvement was observed during outpatient follow-up.
Discussion
Constrictive pericarditis can often be secondary to acute or chronic pericarditis; tuberculous infection being a frequent cause in African and Latin American countries and can also be seen in patients with chronic immunodepression. Other etiologies to consider are postradiation, viral pericarditis, and postpericardiectomy syndrome. Clinical diagnosis of constrictive pericarditis (CP) is difficult and requires a combination of clinical signs and usually more than one cardiovascular imaging technique, such as echocardiography, cardiac catheterization, cardiac computed tomographic angiography (CCTA), or CMR. Patients usually present to the emergency department on multiple occasions with signs and symptoms of CHF, predominantly right-sided, including lower extremity edema, ascites, and hepatomegaly. The main differential diagnosis to be considered is restrictive cardiomyopathy (RC).
Echocardiography is one of the first imaging tests when studying patients with suspected CP, also helping in the differential diagnosis with RC. In patients with CP, LV wall thickness, ventricular dimensions, and systolic function are usually normal, whereas in RC wall thickness may be increased, and LV cavity size reduced. In both CP and RC, left atrial (LA) enlargement is observed due to chronic elevation of LA pressure. M-mode can be useful for detecting abnormal septal movement during diastole (Septal “bounce”) in CP. Pericardial thickening may be evident, as well as dilation of the IVC and hepatic veins. Doppler imaging may show a predominant mitral E wave, with a short deceleration time, in both CP and advanced RC but increased respiratory flow variation is present only in CP and will depend on the volume status. The lateral mitral annulus may be fixed to the pericardium and lateral tissue annular velocity may be lower than the medial tissue annular velocity, known as annulus reversus. An e′>8 cm/s will help to exclude RC. Diastolic reversal flow during expiration may also be seen in the hepatic veins.
CMR is an excellent tool for the evaluation of pericardial thickness. Tissue characterization sequences, such as T2W with fat saturation, are used for the detection of pericardial edema, that may be present in some patients with subacute CP, such as in our patient. LGE is seen and may extend to the entire pericardium. Ventricular interdependence may be very evident in CP, with interventricular septal flattening during inspiration, which can be seen with short-axis real-time sequence. CCTA is especially useful in demonstrating pericardial thickening and calcification.
Once the diagnosis of CP has been made, infectious etiology must be ruled out. Tuberculosis, as mentioned, is one of the most common infectious causes of CP. The pericardial effusion when present, will be typically exudative, with high levels of lymphocytes, proteins, and adenosine deaminase. AFB could be observed in pericardial fluid smears; as well as caseating granulomas in pericardial biopsy.
References
1. Garcia MJ: Constrictive pericarditis versus restrictive cardiomyopathy?. J Am Coll Cardiol 2016; 67: pp. 2061-2076.
2. Welch TD, Ling LH, Espinosa RE, et. al.: Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. Circ Cardiovasc Imaging 2014; 7: pp. 526-534.
3. Adler Y, Charron P, Imazio M, et. al.: 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). G Ital Cardiol (2006) 2015; 16: pp. 702.
4. Geske JB, Anavekar NS, Nishimura RA, Oh JK, Gersh BJ: Differentiation of constriction and restriction. J Am Coll Cardiol 2016; 68: pp. 2329-2347.
5. Klein AL, Abbara S, Agler DA, et. al.: American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013; 26: pp. 965-1012. e15
6. Aldweib N, Farah V, Biederman RWW: Clinical utility of cardiac magnetic resonance imaging in pericardial diseases. Curr Cardiol Rev 2018; 14: pp. 200-212.
7. Isiguzo G, Du Bruyn E, Howlett P, Ntsekhe M: Diagnosis and management of tuberculous pericarditis: what is new?. Curr Cardiol Rep 2020; 22: pp. 2.
A 50-Year-Old Male With Worsening Angina
- Ricardo Oscar Obregón, MD
Synopsis
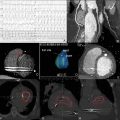
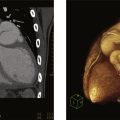
A 50-year-old male, with a prior history of hypertension and prior transient ischemic attack, presented with a 3-month history of progressive angina and worsening functional class from NYHA II to III. On physical examination, he had normal vital signs and a present S4 gallop without murmurs. Basic laboratory test results were normal. Chest X-ray ( Fig. 16.2.1 ) showed normal cardiac silhouette, right pleural effusion, thickening of the minor fissure, and interstitial markings. ECG showed sinus rhythm with complete left bundle branch block. A transthoracic echocardiography (TTE) was performed. Findings ( Fig. 16.2.2 ) were consistent with regional wall motion abnormalities and a calcified LV mass. The patient was referred for cardiac magnetic resonance (CMR) ( Fig. 16.2.3 ), which confirmed the presence of regional wall motion abnormalities in the circumflex distribution and signal void suggestive of myocardial calcification. Cardiac computed tomographic angiography (CCTA) ( Fig. 16.2.4 ) confirmed myocardial calcification extending into the LV cavity and anomalous origin of the left coronary artery from the pulmonary trunk. The diagnosis was confirmed by invasive coronary angiography ( Fig. 16.2.5 ). A summary of the noninvasive imaging findings is provided in Table 16.2.1 .





| Modality | Summary of Findings | Figure No. |
|---|---|---|
| Chest X-ray | AP and lateral films show normal cardiothoracic index and clean lung fields. No flow redistribution is observed. Within the cardiac area, hyperintensity is compatible with calcifications within the cardiac silhouette, normal cardiac silhouette, right pleural effusion, thickening of the minor fissure, and interstitial markings. | Fig. 16.2.1 |
| TTE | The 4-chamber image showed a hyper refringent image, with a calcified appearance in the lateral wall of the LV from where a fine and very mobile filamentous image is projected that prolapses into the LV cavity. Other findings in TTE included regional wall motion abnormalities in the mid-lateral wall with an LV EF = 51%. | Fig. 16.2.2 |
| CMR | With the cine sequence of gradients in a two-chamber section, basal and medial anterior akinesia can be seen, with a hypointense image on the anterior face compatible with fibro/calcified structure in the wall. | Fig. 16.2.3A |
| With fast field echo cine sequences in multiple ventricular short-axis slices, anterior akinesia can be seen with hypointense images that grow from the ventricular cavity, entering the myocardial muscle in the form of ramifications (possible classified coronary pathways). | Fig. 16.2.3B | |
| Degraded cine image (FFE) in a four-chamber section, lateral akinesia can be seen with a circumscribed area of hypo intensity, possibly calcium. | Fig. 16.2.3C | |
| CCTA | Noncontrast scan shows large, calcified areas located at the atrioventricular groove and in the vicinity of the LCx artery. | Fig. 16.2.4A |
| With contrast, calcification extents to papillary muscles. The image demonstrates large caliber RCA | Fig. 16.2.4B | |
| Volumetric reconstruction of left main coronary artery arising from the lower portion of the pulmonary artery. | Fig. 16.2.4C | |
| ICA | Contrast injection in the RCA shows retrograde filling of the LAD that drains into the trunk of the pulmonary artery. | Fig. 16.2.5A |
| The ventriculogram shows anterior basal and medial akinesia. | Fig. 16.2.5B |
Discussion
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital coronary abnormality associated with early infant mortality and adult sudden death. Our 50-year-old patient came to the office due to progressive dyspnea and angina. The TTE and CMR demonstrated findings consistent with a remote myocardial infarction with myocardial calcification. Cardiac computed tomography (CCT) showed a dilated right coronary artery (a common finding in ALCAPA) with an anomalous origin of the left main arising from the main pulmonary artery.
References
1. Keith JD: The anomalous origin of the left coronary artery from the pulmonary artery. Br Heart J 1959; 21: pp. 149-161.
2. Frommelt P, Lopez L, Dimas V, Eidem B, Han K, Ko H, et. al.: Recommendations for multimodality assessment of congenital coronary anomalies: a guide from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2020; 33: pp. 259-294.
A 78-Year-Old Male With Acute Chest Pain
- Lilia Mercedes Sierra-Galan, MD
- Fernanda Trejo-Millan, MD
- Jorge Luis Denis-Bravo, MD
- Fernanda Trejo-Millan, MD
Synopsis
A 78-year-old male with a history of hypertension and coronary artery disease status post percutaneous coronary intervention x 3 (1994, 2005, 2018 with unknown anatomy) was admitted to the emergency room with acute chest pain at rest with spontaneous remission after 15 minutes. However, after 24 hours, he presented a new episode of chest pain and decided to seek medical attention. His ECG showed sinus bradycardia without evidence of myocardial ischemia. Laboratory findings showed mildly elevated troponin levels.
A stress cardiac magnetic resonance (CMR) protocol with a dedicated acute ischemia protocol consisting of a fast CMR acquisition was performed ( Fig. 16.3.1 ). It showed mild left ventricular global systolic dysfunction, with LVEF of 56% and subtle wall motion abnormalities in the left anterior descending (LAD) and circumflex (LCx) territories ( Fig. 16.3.2 and ![]() ). Stress first-pass perfusion with intravenous adenosine infusion at 140 µg/kg/min per 6 minutes showed severe myocardial ischemia in the LAD and LCx territories ( Fig. 16.3.3 and
). Stress first-pass perfusion with intravenous adenosine infusion at 140 µg/kg/min per 6 minutes showed severe myocardial ischemia in the LAD and LCx territories ( Fig. 16.3.3 and ![]() ) with a small subendocardial late gadolinium enhancement (LGE) in the basal anterior segment corresponding to an old myocardial subendocardial infarction in the LAD territory ( Fig. 16.3.4 ). In addition, the patient had retro-sternal chest pain and premature ventricular beats during the adenosine infusion.
) with a small subendocardial late gadolinium enhancement (LGE) in the basal anterior segment corresponding to an old myocardial subendocardial infarction in the LAD territory ( Fig. 16.3.4 ). In addition, the patient had retro-sternal chest pain and premature ventricular beats during the adenosine infusion.




Based on the CMR, the patient was taken to the catheterization laboratory. Invasive coronary angiography (ICA) ( Fig. 16.3.5 ) showed significant distal left main stenosis extending to the ostial LAD and LCx. Therefore, a percutaneous coronary intervention was performed with successful stent deployment in the proximal left main, LAD, and LCx. A summary of the noninvasive imaging findings is provided in Table 16.3.1 .

Discussion
Stress perfusion CMR is a sensitive, specific, and accurate noninvasive method to detect abnormalities in the coronary artery system and its blood flow reserve. Also, it can provide short- and long-term prognostic information and allows risk stratification of patients with suspected coronary artery disease. There are two methods to induce myocardial stress perfusion including exercise and pharmacological (including vasodilators and positive inotropic drugs such as dipyridamole, adenosine, regadenoson, and dobutamine). A popular protocol recommends the administration of adenosine at a dose of 140 µg/kg/min and increased up to 210 µg/kg/min if required.
Commonly, stress CMR is used for stable patients with suspected or known coronary artery disease. Still, some projects have studied the clinical scenario of acute chest pain in the emergency department using adenosine. Using this medication as a stressor, CMR has been reported to identify perfusion defects with 100% sensitivity and 93% specificity when combined with function and LGE analysis, showing that CMR is an accurate imaging modality for the identification of myocardial ischemia.
This case illustrates the feasibility of stress perfusion CMR. It highlights the relevance of this imaging modality in identifying coronary artery disease with important prognostic information, as has mainly been demonstrated by key studies such as the CE-MARC, a large, prospective, and real-world trial for the evaluation of stress CMR that established its high diagnostic accuracy compared to SPECT.
The 2021 chest pain guidelines from the AHA, the ACC, the ASE, the CHEST, the SAEM, the SCCT, and the SCMR recommend using CMR in acute and stable chest pain with class 1 or 2a of recommendation in several different scenarios. Therefore, CMR may be considered more often in the routine clinical scenario.
References
1. García-Baizán A, Millor M, Bartolomé P, et. al.: Adenosine triphosphate (ATP) and adenosine cause similar vasodilator effects in patients undergoing stress perfusion cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2019; 35: pp. 675-682.
2. Biko DM, Collins RT, Partington SL, et. al.: Magnetic resonance myocardial perfusion imaging: safety and indications in pediatrics and young adults. Pediatr Cardiol 2018; 39: pp. 275-282.
3. Brown LAE, Saunderson CED, Das A, et. al.: A comparison of standard and high dose adenosine protocols in routine vasodilator stress cardiovascular magnetic resonance: dosage affects hyperaemic myocardial blood flow in patients with severe left ventricular systolic impairment. J Cardiovasc Magn Reson 2021; 23: pp. 37.
4. Ingkanisorn WP, Kwong RY, Bohme NS, et. al.: Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol 2006; 47: pp. 1427-1432.
5. Greenwood JP, Maredia N, Younger JF, et. al.: Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012; 379: pp. 453-460.
6. Greenwood JP, Ripley DP, Berry C, et. al.: Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or nice guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 randomized clinical trial. JAMA 2016; 316: pp. 1051-1060.
7. Nagel E, Greenwood JP, McCann GP, et. al.: Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 2019; 380: pp. 2418-2428.
8. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285.
A 78-Year-Old Male With Recurring Episodes of Abdominal-Chest Pain
- Carlos Andres Rojas, MD
Synopsis
A 78-year-old male with a history of hypertension, hyperlipidemia, active smoking, diabetes, and coronary artery disease [a history of coronary calcium score performed 10 years ago showed calcified plaque in the left main and left anterior descending (LAD)] presented to the emergency department due to recurring episodes of abdominal-chest pain in the last week. Episodes of pain lasted 10–15 minutes, localized in the epigastrium, and were quantified as 8–9/10. On physical exam, the patient was afebrile with normal vital signs. Troponin was elevated without significant delta (30 ng/L on admission, 36 ng/L at 2 hours, and 28 ng/L at 6 hours with reference range < 15 ng/L). ECG showed sinus bradycardia without ST elevation or depression and the Heart score was 5. Transthoracic echocardiography showed a calcified aortic valve with mild aortic stenosis and normal biventricular size and systolic function. CCTA with fraction Flow reserve (CT-FFR) was performed for further evaluation ( Fig. 16.4.1 ). Subsequent invasive coronary angiography ( Fig. 16.4.2 ) showed a 90% discrete lesion in the proximal LAD and a 30% lesion in the second diagonal branch. The patient underwent successful percutaneous coronary intervention with stent placement in the LAD. A summary of the noninvasive imaging findings is provided in Table 16.4.1 .


| Modality | Summary of Findings | Figure No. |
|---|---|---|
| CTA | Prospectively triggered Coronary CT angiography axial dataset demonstrates a large amount of predominantly calcified plague in the proximal to mid-LAD with moderate stenosis (CAD-RADS 3). Nondiagnostic evaluation of the mid RCA due to motion. | Fig. 16.4.1A |
| Coronary CT angiography demonstrates a large amount of predominantly calcified plaque in the proximal mid-LAD with moderate stenosis. | Fig. 16.4.1B | |
| CT-FFR evaluation demonstrates a high likelihood of flow-limiting stenosis in the mid-LAD lesion and downstream diagonal branch. | Fig. 16.4.1C | |
| ICA | Pre (A) and post (B) invasive coronary angiogram before and after percutaneous coronary angioplasty of the mid-LAD 90% lesion. | Fig. 16.4.2 |
Discussion
In the setting of a 78-year-old male with known coronary artery disease presenting with acute chest pain and elevated troponin, several testing modalities are acceptable for risk stratification. At our institution given, our experience and availability, a CCTA followed by CT-FFR was performed. According to the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guidelines, CCTA is a class 2a recommendation with level B-NR quality of evidence for the evaluation of patients with acute chest pain, known coronary artery disease, and intermediate risk for acute coronary syndrome. Similarly, CT-FFR is a class 2a and level B-NR for the evaluation of 40%–90% coronary stenosis in a proximal or middle coronary segment on coronary CT angiography. Our imaging protocol consists of a prospectively triggered calcium score followed by a prospectively triggered cardiac CT angiogram after the administration of intravenous contrast (100 cc of Iohexol 350 mg/ml) at an injection rate of 5 cc/s. Heart rate control is performed using oral beta-blockers for heart rate control and coronary vasodilation is performed using sublingual nitroglycerin spray. Coronary CT angiography images demonstrated a large amount of predominantly calcified plaque in the proximal to mid-LAD which can limit the accurate evaluation of the lumen. It was estimated that the degree of luminal narrowing was in the 50%–69% range (CAD-RADS 3) and therefore a candidate for further evaluation with CT-FFR. CT-FFR showed the proximal LAD lesion to be hemodynamically significant (<0.80) and therefore invasive coronary angiography was performed. On invasive coronary angiography, the LAD stenosis was confirmed (90% discrete lesion) and the patient underwent successful angioplasty and stent placement.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree