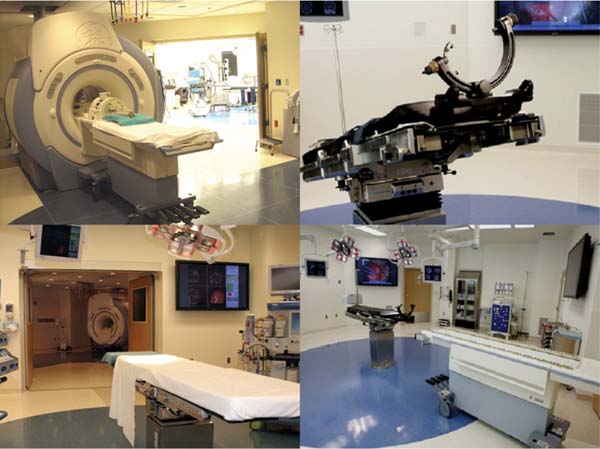
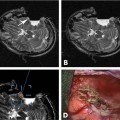
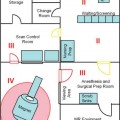
19 The surgical treatment of lesions involving the cranial base has evolved significantly over the past several decades. As in other neurosurgical disciplines their surgical management has relied heavily upon advances in technology and imaging modalities. Surgical adjuncts such as high-powered microscopes, ultrasonic aspirators, and endoscopes have refined skull base surgery techniques. Nerve stimulation and motor and sensory evoked potential monitoring provide invaluable electrophysiologic information regarding anatomy as well as the progress and effects of surgery. High-resolution computerized tomographic imaging (CT), magnetic resonance imaging (MRI), and advanced angiographic systems and techniques have greatly refined the preoperative localization and anatomic understanding of many of these lesions, particularly with regard to bony, soft tissue and neurovascular relationships. The development of computerized surgical navigation systems has had tremendous impact upon many aspects of skull base surgery.1–3 Based upon preoperatively acquired imaging, these image guidance systems are used to plan more refined surgical approaches to lesions and craniotomies that are smaller and more directly over the target. Intraoperatively, real-time image-based guidance allows the surgeon to accurately approach the lesion and provides constant information on the progress of surgery as well as the anatomic relationships of the various surrounding structures. This is of particular importance in skull base lesions where eloquent structures such as brainstem and/or cranial nerves are often incorporated within or are in very close proximity to a lesion and normal anatomic relationships are frequently distorted or destroyed. The development of intraoperative magnetic resonance imaging (iMRI) systems over the past two decades has provided a unique tool for the surgeon to evaluate and assess the effects and progress of a surgical procedure. A variety of iMRI systems have been developed by different vendors in conjunction with surgeons throughout the world.4–11 These systems differ vastly from one another in several characteristics. The main differences are magnet field strength (ranging from 0.12 tesla [T] to 3.0 T), whether the surgery is performed within the magnetic field, and whether the patient is transported to the magnet or whether the magnet is transported to the patient for imaging. Despite this heterogeneity of iMRI systems, it has become evident that the acquisition of high-resolution MR imaging and advanced MRI techniques require higher-field systems,9,12 which allow the MRI evaluation of the anatomic and when warranted, the physiologic effects of surgery. Furthermore, by updating image-guidance systems with iMRI data, the inaccuracies in stereotaxy induced through operation and surgical resection can be corrected and residual lesion precisely localized.13,14 Skull base lesions, given their relationships to complex but relatively constant anatomic structures represent unique lesions with particular potential to benefit from iMRI. The operations on skull base lesions discussed in this report were performed at Barrow Neurologic Institute (BNI) in Phoenix, Arizona. The iMRI system at the BNI utilizes a GE Signa HDx (General Electric Medical Systems, Waukesha, WI) 3.0 T MRI unit. The magnet is identical to the commercially available diagnostic unit marketed by GE. The magnet has a working aperture of 60 cm and weighs 12,000 kg. It is equipped with high power 23 to 50 mT/m gradients. Slew rate is 80 to 150 mT/m per millisecond. The magnet is 2.4 m tall, 2.2 m wide, and 2 m in length. The 5 gauss (G) fringe field is 2.8 × 5.0m, well within the confines of the imaging suite. The electronic operating room (OR) table is a specially designed unit by Maquet (Maquet GmbH, Rastatt, Germany) and possesses all of the positional capabilities of a standard OR table as well as a specially designed MRI-compatible skull fixation attachment. The iMRI system is located in a suite within the main neurosurgical operating room area and is directly accessible from two adjacent, connecting ORs (Fig. 19.1). For iMRI the patient is transported, anesthetized, and intubated into the MRI scanner. Only patients undergoing surgery in either of the two directly adjacent rooms can undergo iMRI while remaining in skull fixation and the surgical site open. Although patients in the remaining rooms must have their wound closed and be removed from skull fixation prior to being transported for imaging, all 11 neurosurgical ORs have access to the MRI scanner for intraoperative imaging. For a variety reasons including patient positioning restrictions due to patient size, instrumentation and magnet working aperture, time restrictions, etc., the majority of iMRI performed is with the surgical wound closed and the patient removed from skull fixation. The OR equipment and room is always maintained sterile for potential return for further surgery following iMRI. Frameless stereotaxy is utilized in all cases. The Stealth Treon (Medtronic Navigation, Inc., Louisville, CO) stereotactic neuronavigation system is available in all the ORs and is closely integrated with the surgical microscopes. iMR images can be readily transferred to the Stealth system and are utilized to update the navigation system to correct for brain and lesion deformation and/or shift as a result of surgery. Accuracy has been well maintained during this data transfer and re-registration with iMR images. Fig. 19.1 Top left: GE 3.0 tesla intraoperative magnetic resonance imaging (iMRI) system (General Electric Medical Systems, Waukesha, WI) with one of directly accessible two adjacent operating rooms (ORs) in the background. Gantry is docked to magnet in imaging position. Bottom left: View from other OR with doors leading to iMRI suite open. Operating table in foreground. Top right: Maquet OR table (Maquet GmbH, Rastatt, Germany) with MRI-compatible head holder. Bottom right: The mobile MR gantry preparing to dock with OR table for transfer to iMRI suite. As the magnetic field within the operating room is negligible, all surgical procedures were performed using standard microneurosurgical instruments, equipment and techniques. This included use of ultrasonic aspiration, CO2 laser, endoscope, standard high-powered surgical microscope, etc., as needed. A Zeiss Pentero (Carl Zeiss AG, Oberkochen, Germany) microscope with surgical chair and foot and mouth controls was utilized for all operations. Electrophysiologic monitoring including somatosensory evoked potentials (SSEPs) and/or any other applicable modalities, such as brainstem auditory evoked potentials (BAERs) and cranial nerve monitoring, were utilized as deemed necessary by the surgeon. All monitoring leads were removed for iMRI. All iMR images were obtained with the patient intubated and anesthetized. For transfer to the imaging suite ventilation was applied by the anesthesiologist by bag-mask. For iMRI with the patient remaining in skull fixation with the wound exposed (n =4), all non-MR-compatible leads and instruments are removed and a draping protocol is utilized to maintain sterility of the surgical site. The patient is then transferred with the mobile tabletop on to the mobile MR gantry and transported to the iMR suite for MRI. In the MR suite an MR-compatible ventilator is utilized during imaging. Following MRI, the patient is transported back to the OR and the tabletop and patient are transferred back to the OR table. The sterile draping is unfolded and the surgery site reexposed, navigational data transferred to the Stealth system, and surgery resumed. All standard MRI sequences were available and obtained as requested by the surgeon. These included T1-weighted, T2-weighted, fluid attenuation inversion recovery (FLAIR), gradient echo, gadolinium-enhanced, diffusion-weighted, and MR angiography. Average imaging time for this group of patients was 55 minutes. The surgical treatment of a broad range of skull base pathologies was monitored utilizing iMRI. Included in this series were any lesions involving the following regions: sellar/parasellar, cavernous sinus, orbit, clivus, nasopharynx, petrous bone, and posterior fossa (n = 68, see Table 19.1).
Skull Base Surgery and Intraoperative Magnetic Resonance Imaging
The Intraoperative MRI System
Clinical Experience
Surgical Technique
Imaging Studies
Surgery of Skull Base Pathology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree