Currently, the worldwide number of tobacco smokers is estimated at 1.1 billion people, with the World Health Organization estimating that tobacco use is responsible for approximately 6 million premature deaths each year. In the United States 42 million adults are still smokers despite overall decreasing rates of smoking in the United States. Despite a majority of the morbidity and mortality of smoking being related to lung cancer, coronary atherosclerosis, and chronic obstructive pulmonary disease, the smoking-related interstitial lung diseases are a commonly encountered and important clinical and radiologic entity.
The smoking-related interstitial lung diseases include a range of conditions, from those highly associated with smoking, such as respiratory bronchiolitis (RB), desquamative interstitial pneumonia (DIP), and pulmonary Langerhans cell histiocytosis (PLCH, see Chapter 33 ), to other conditions that are worsened or precipitated by smoking, such as acute eosinophilic pneumonia (AEP, see Chapter 37 ), pulmonary hemorrhage (see Chapter 2 ), and even pulmonary fibrosis. This chapter focuses on the smoking-related interstitial pneumonias (RB, respiratory bronchiolitis–associated interstitial lung disease [RB-ILD], and DIP) and the smoking-related patterns of pulmonary fibrosis, including usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), and combined pulmonary fibrosis and emphysema (CPFE).
Respiratory Bronchiolitis and Respiratory Bronchiolitis–Associated Interstitial Lung Disease
Etiology
RB is a common histopathologic finding in smokers, which typically is not associated with symptoms or functional deficit. A few smokers develop symptoms and are classified as having RB-ILD. RB-ILD is a symptomatic clinical condition with radiologic and functional abnormalities that was first recognized in heavy cigarette smokers in the late 1980s. Subsequent studies have confirmed a strong link with cigarette smokers, with very rare reports in nonsmokers, although a link with inhalation of noxious substances in the workplace also has been suggested.
Prevalence and Epidemiology
Despite the common habit of cigarette smoking in the general population and the frequency of RB in smokers approaching 100% in some studies, the clinicopathologic entity of RB-ILD is comparatively rare, making accurate evaluation of prevalence difficult. RB-ILD was not yet a recognized disease in the earlier large milestone multicenter studies of interstitial lung disease that predated the 1990s. In a study from a large tertiary referral center in the United Kingdom, the biopsy specimens in 168 cases over an 18-year period were retrospectively reviewed, and 13 (8%) of these showed a dominant pattern of RB-ILD. The usual age at presentation is in the 30s and 40s, and men and women are almost equally affected, with a slight male preponderance.
Clinical Presentation
Patients with RB-ILD are invariably middle-aged smokers and present with dyspnea that may be of insidious onset. Cough also may be a feature and is sometimes severe. Less commonly, chest pain and, rarely, hemoptysis have been described. Bilateral end-inspiratory crackles, which may be predominantly basal, are common, but finger clubbing is very rare.
Pathophysiology
Pathology
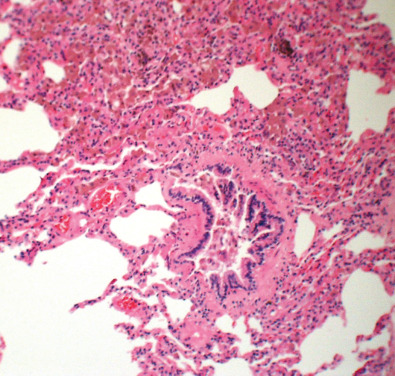
RB is characterized histologically by intraluminal and peribronchiolar airspace accumulation of pigmented macrophages ( Fig. 34.1 ). These macrophages are called “smoker’s macrophages” because they typically have granular brown cytoplasm. The pigmentation most likely represents the metabolites of cigarette smoke, given that the intensity of pigmentation correlates with pack-years of cigarettes smoked. An ongoing inflammatory process is denoted by a mild peribronchiolar mononuclear inflammatory submucosal infiltrate, which may be associated with fibroblasts and collagen deposition, resulting in stellate fibrous scarring extending into surrounding alveolar walls. Similar to macrophage pigmentation, peribronchiolar fibrosis also has been shown to correlate with the number of pack-years smoked. Additional histopathologic findings include thickening of the peribronchiolar alveolar septa and alveolar ducts and histiocytes within the peribronchiolar fibrous tissue displaying anthracotic pigment, foci of goblet cell metaplasia, and metaplastic cuboidal epithelium in the airway epithelium. Mild emphysema in the surrounding lung parenchyma is relatively common, but honeycombing is not a recognized histopathologic feature and should raise the possibility of alternative diagnoses, with RB being an incidental finding.
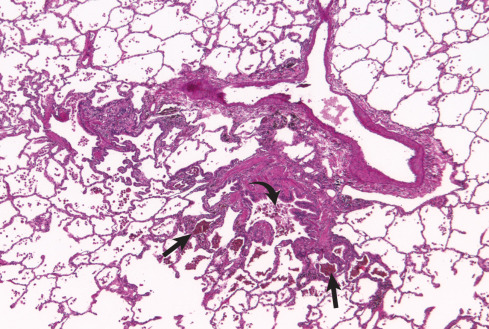
Histopathologic findings in RB-ILD ( Fig. 34.2 ) and DIP are similar, and differentiation between the two entities may be difficult. The distribution in RB-ILD is typically more patchy and bronchiolocentric, whereas DIP results in extensive uniform alveolar macrophage accumulation. Currently, RB-ILD and DIP are considered to be a spectrum of smoking-related parenchymal disease that also may include PLCH. Correlation with clinical and radiologic findings is vital in the diagnosis of RB-ILD, owing to the almost universal, usually incidental, histopathologic observation of RB in asymptomatic smokers.
Lung Function
Individuals with uncomplicated RB usually do not have lung function deficits. In contrast, the usual pulmonary function findings in RB-ILD are a mixed obstructive-restrictive defect. The carbon monoxide–diffusing capacity is often reduced, and the total lung capacity may be normal, reduced, or slightly increased. In one study the most common functional deficit was a reduction in diffusing capacity. There is an inverse correlation between the computed tomography (CT) extent of ground-glass opacity and arterial oxygen saturation.
Manifestations of the Disease
Radiography
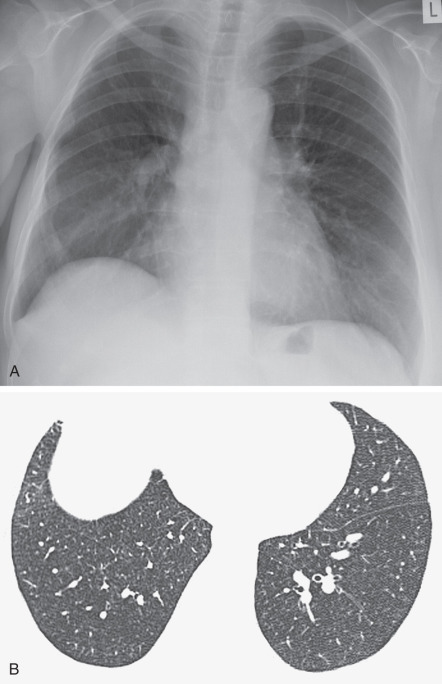
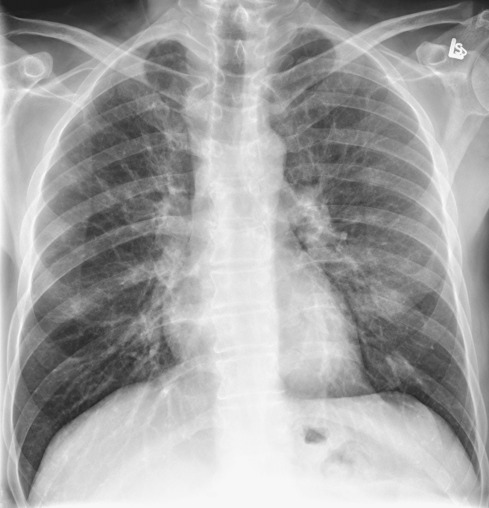
The radiographic features of biopsy-proven cases of RB-ILD have been described in numerous studies and are variable and nonspecific. A fine diffuse reticulonodular pattern with normal lung volumes has been described ( Fig. 34.3 ). In one study the main finding was the presence of ill-defined small nodules with lower zone predominance. In another study the main abnormalities consisted of ground-glass opacities ( Fig. 34.4 ) and airway wall thickening, with no cases showing a prominent reticulonodular pattern. One-third of patients may have a normal radiograph, despite biopsy-proven disease.
Computed Tomography
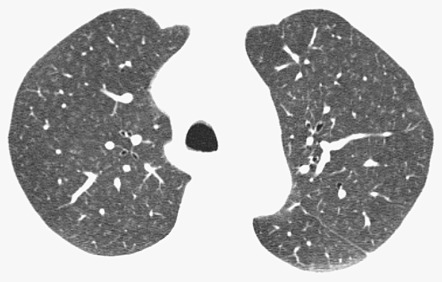
Comparison of the high-resolution CT (HRCT) findings in 98 asymptomatic cigarette smokers with 175 nonsmoking controls showed ill-defined, upper zone–predominant parenchymal micronodules in 27% of smokers but not in control subjects. Comparable differences were noted for areas of ground-glass opacity (20% of smokers and none of the control group). In a later study of heavy smokers with histopathologic correlation, parenchymal micronodules indicating the presence of RB ( Fig. 34.5 ) and areas of ground-glass opacification, representing accumulations of inflammatory cells and a variable degree of fine interstitial fibrosis, were observed. In another study investigating CT features in 57 smokers at baseline and follow-up examinations, the percentages of patients with ground-glass opacity, emphysema, and ill-defined micronodules increased, respectively, from 28% to 42%, 26% to 40%, and 33% to 35% on follow-up. Five patients displaying micronodules at baseline examination showed a replacement of this pattern by emphysema at follow-up.
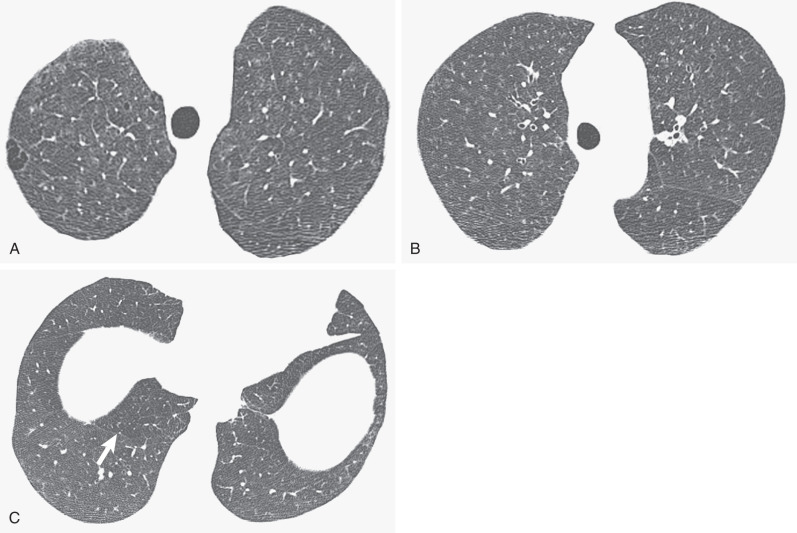
The HRCT findings of RB-ILD were initially described in five patients with biopsy-proven diagnosis. Areas of ground-glass opacity ( Fig. 34.6 ) were the most common finding. One patient had interlobular and intralobular thickening ( Fig. 34.7 ). No patient had nodular opacities. By contrast, a more recent study of 21 patients with pathologically proven RB-ILD documented centrilobular nodules in 71% of cases (see Fig. 34.7 ), areas of ground-glass opacity in 67%, and patchy areas of decreased attenuation (likely reflecting small airways disease) in 38%. End-expiratory CT may emphasize the regional differences in lung density ( Fig. 34.8 ). Most patients in the latter study showed wall thickening of proximal and distal airways, a similar appearance to that seen in chronic bronchitis. In another series of eight patients with RB-ILD, no single HRCT finding predominated. Diffuse ground-glass opacification and centrilobular nodules were major features in about half of the patients, and emphysema was present in most patients. Despite the fact that many patients are heavy smokers, the severity of the emphysema is usually mild or trivial, confined to the upper lobes, and with a centrilobular or paraseptal distribution. Some patients show thickening of interlobular septa, and features of established interstitial fibrosis are unusual but coexist in some patients with RB-ILD. A mosaic attenuation pattern, representing constrictive bronchiolitis, is not usually a prominent feature, but when present, it is most obvious in the lower lobes. The combination of HRCT features of an infiltrative and small airways disease is similar to that seen in patients with subacute hypersensitivity pneumonitis (HP), and the distinction between these two conditions often rests on the smoking history because hypersensitivity pneumonitis is uncommon in smokers. The HRCT appearances of RB-ILD are variable and nonspecific. The CT features of RB are often subtle.

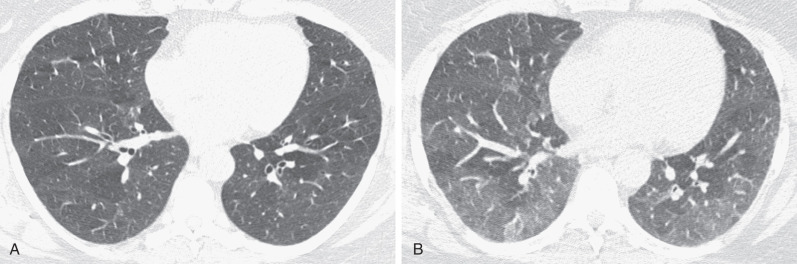
Imaging Algorithms
The chest radiograph may be normal in RB and RB-ILD. When the radiograph is abnormal, the findings are often nonspecific. HRCT is more sensitive, especially in subtle disease.
Differential Diagnosis
The differential diagnosis based on clinical data includes other diffuse interstitial lung diseases, especially those related to smoking (e.g., desquamative interstitial pneumonia). The HRCT appearance of RB-ILD is very similar to HP. The history of smoking can be very useful to distinguish between RB-ILD and subacute HP. Additionally, bronchoalveolar lavage demonstrating smokers’ macrophages and a lack of lymphocytosis is strongly suggestive of RB-ILD and may prevent the need for surgical lung biopsy in a majority of patients when combined with appropriate HRCT findings.
Synopsis of Treatment Options
- •
The prognosis seems to be improved in individuals with RB-ILD who stop smoking, although persisting radiologic and functional abnormalities may be seen.
- •
The response to corticosteroids is unclear; most patients report a symptomatic improvement, which may be associated with improvement of lung function indices but may not result in complete disease resolution.
- •
Respiratory bronchiolitis (RB) is a common histopathologic finding in smokers but is not usually associated with symptoms.
- •
Respiratory bronchiolitis–interstitial lung disease (RB-ILD) is a rare symptomatic interstitial lung disease linked with cigarette smoking and part of the spectrum of smoking-related interstitial lung disease.
- •
RB and RB-ILD have nonspecific radiographic features, such as reticulonodular pattern, bronchial wall thickening, and ground-glass opacities. Radiographs may be normal.
- •
High-resolution CT (HRCT) usually shows poorly defined centrilobular nodules and ground-glass opacities with an upper zone predominance. There may be evidence of emphysema.
- •
HRCT features of RB-ILD may be subtle and similar to HP.
Desquamative Interstitial Pneumonia
Etiology
Desquamative interstitial pneumonia (DIP) is a rare condition that is strongly linked with cigarette smoking. DIP was first described by Liebow in 1965 and was initially thought to be an early “cellular” or inflammatory phase of idiopathic pulmonary fibrosis (IPF). Subsequent clinical and radiologic longitudinal studies have shown, however, that the prognosis and natural history of the condition vastly differ from the inexorable decline invariably seen in patients with IPF. Similarly, the predominant histopathologic pattern was initially thought to be that of desquamated pneumocytes (hence the term desquamative interstitial pneumonia ) but subsequently was recognized as widespread alveolar filling with macrophages. The original term continues to be used despite it being a now recognized misnomer. Despite the fact that DIP is currently classified as an “idiopathic” interstitial pneumonia, greater than 90% of cases of DIP are in smokers, and the condition is considered to represent part of the histopathologic spectrum of smoking-related interstitial lung diseases, including PLCH and RB-ILD. A “DIP-like” histopathologic pattern also has been associated with dust inhalation, metabolic diseases, drug reactions, and connective tissue diseases. DIP has been described in children as well. In infants a similar pattern has been described that is related to mutations in the surfactant protein C gene, a familial condition that is unrelated to cigarette smoke exposure.
Prevalence and Epidemiology
DIP as a “pure” entity is extremely rare, and accurate data on the prevalence are not reliable. There is a male-to-female ratio of 2 : 1 in DIP, and 90% are smokers. Individuals usually present in their 30s to 50s. DIP has been reported in children and, although rare, is one of the more common histopathologic patterns of interstitial lung disease in this age group.
Clinical Presentation
Patients usually present with insidious onset of dyspnea and nonproductive cough. On clinical examination more than half of patients have crepitations (i.e., lung crackles), and approximately 50% display finger clubbing, which is not normally seen in RB-ILD.
Pathophysiology
Pathology
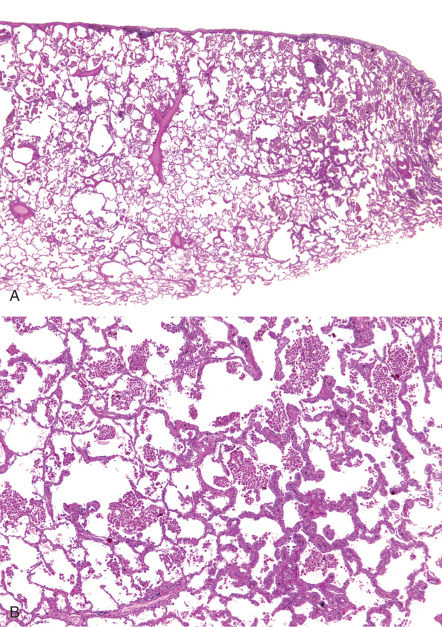
The histopathologic features of DIP are similar to RB-ILD in that there is pigmented macrophage accumulation in alveolar spaces. The discriminating factor between these conditions is the extent of involvement, with RB-ILD being typically patchy and bronchiolocentric, whereas DIP is more uniform and diffuse, a feature that can be recognized at low magnification ( Fig. 34.9 ). Despite the apparently straightforward difference, there is overlap in appearances between these two conditions, making definitive histopathologic diagnosis problematic in some cases. Eosinophils may be seen in addition to macrophages, a feature not normally seen in RB-ILD. The background architecture of the alveoli is generally well maintained; however, the alveolar septa may be minimally thickened owing to inflammatory cells and fibrosis, a more extensive feature than in RB-ILD. Extensive fibrosis with honeycomb change is not a feature usually associated with DIP, compared with UIP, although mild honeycombing may occur.