12
Soft Tissue Sarcoma Brachytherapy
Caroline L. Holloway and Chandrajit P. Raut
Soft tissue sarcomas (STS) are an uncommon malignancy that can present throughout the body including the extremities, head and neck, retroperitoneum, trunk, and skin. Surgery is the primary treatment while radiation therapy (RT) and chemotherapy are adjuvant treatment options.
The use of RT in combination with wide local excision (WLE) for extremity STS has enabled functional limb preservation with acceptable local control. The most common RT techniques include pre- or postoperative external beam radiotherapy (EBRT), brachytherapy monotherapy or brachytherapy in combination with EBRT. Brachytherapy in the treatment of STS has been reported in the literature since the 1970s and now includes reports on low dose rate (LDR), pulsed dose rate (PDR), and high dose rate (HDR) techniques. No prospective trials have directly compared brachytherapy and EBRT for either primary or recurrent STS.
PATIENT SELECTION
Review by a multidisciplinary sarcoma team to discuss pathology, imaging, and management is recommended for all patients. Patients with small (less than 5 cm) tumors that can be resected with clinically and pathologically appropriate margins (greater than 1 cm or intact fascia or other biologic barriers) are candidates for surgery alone (1,2). Radiation as an adjuvant treatment is recommended if there is a high risk of local recurrence, including close or positive surgical margins, deep tumors greater than 5 cm, high grade, local recurrence after prior surgery, and age less than 50 years (3). The American Brachytherapy Society (ABS) consensus statement supports brachytherapy as monotherapy in patients with high-grade STS of the extremity or superficial trunk with negative margins and brachytherapy in combination with EBRT for cases with recurrent disease who have not previously been irradiated (4). The location of the tumor may also play a factor in management decisions; for example, some studies have shown an increased risk of recurrence for sarcomas located in the upper extremity and shoulder region (5,6).
TREATMENT RESULTS
Brachytherapy Monotherapy
Low Dose Rate
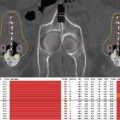
Only one prospective randomized controlled trial has evaluated the role of brachytherapy in STS. Investigators at Memorial Sloan-Kettering Cancer Center (MSKCC) randomized patients with extremity STS who had undergone a macroscopically complete resection (R0, with negative microscopic margins, or R1, with positive microscopic margins) to receive either postoperative catheter-based brachytherapy or no additional adjuvant therapy. The trial utilized LDR brachytherapy as a monotherapy with a planned radiation dose of 42 to 45 Gy over 4 to 6 days. No patients received EBRT. This study showed local control (LC) to be significantly improved with brachytherapy compared with surgery alone, but only in patients with high-grade STS (69% vs 82% 5-year LC, P = .04); there was no difference in patients with low-grade tumors. There was no difference in rates of distant metastases and no improvement in disease-free survival (DFS) or overall survival (OS) rates (7). Other reports in the literature using LDR brachytherapy alone showed local control rates between 75% and 90% (Table 12.1) (6,8–11).
Brachytherapy departments are increasingly favoring HDR or PDR techniques secondary to availability, ability to optimize treatment plans, decreased radiation exposure to staff, and minimal need for patient isolation. There are no randomized comparisons of LDR, HDR or PDR treatments for brachytherapy for STS.
High Dose Rate
There are no randomized trials of HDR brachytherapy in STS. More frequently, however, HDR is being reported and used in STS. Reports of local control range between 50% and 92% (12–15).
Pulsed Dose Rate
Reports using PDR brachytherapy are minimal in STS. Lazzaro et al reported their experience of 34 patients treated with either PDR brachytherapy alone (n = 18) or in combination with EBRT (n = 16). The local control rates were comparable between the monotherapy arm (88%) and the combined arm (92%) (16).
Table 12.1 Sarcoma monotherapy

Brachytherapy in Combination With EBRT
The impact of brachytherapy in combination with EBRT has not been evaluated prospectively (Table 12.2). Local control rates for LDR or HDR brachytherapy in combination with EBRT range from 71% to 100% (Table 12.3) (8,9,11–13,17–28). There is no consensus as to which patients should be treated with brachytherapy and EBRT. Some reports from both the LDR and HDR literature have suggested a local control advantage to EBRT plus brachytherapy for tumors with positive margins (14,15,27), while others have not (29). Other variables that have been associated with improved local control with combined EBRT and brachytherapy include high grade, large size, and recurrent disease (12,15,23).
TOXICITY
The added complication rate for brachytherapy for STS treatment is difficult to quantify as the treatments usually involve multiple therapeutic modalities. Complication probabilities may relate not only to adjuvant brachytherapy but also to EBRT, use of chemotherapy, extent of resection, tumor size, proximity to nerves, vessels, and skin, and use of skin grafts or flaps.
The most common acute complication is delayed wound healing. In the MSKCC randomized trial, the wound complication rate was not significantly different between the surgery-alone arm and the surgery and brachytherapy arm (14% vs 24%, P = .13). There was a higher reoperation rate, however (0% vs 10%, P = .006). The rate of wound complications in the literature ranged from 7% to 59% (9,10,12,15,16,27,28,30), and the rate of reoperation was between 2.3% and 13.8% (27). The time to source loading was shown to impact on the acute toxicity; delaying catheter loading until the fifth postoperative day reduced the postoperative complication rate (31). For LDR techniques, the implant geometry and the number of needles correlate with higher rates of acute toxicity (9,28). In contrast, the toxicity rates with HDR brachytherapy are related more to the total dose of radiation given, brachytherapy fraction size, and the volume receiving 150% of the prescription dose (V150%) (25).
Techniques to minimize acute toxicity include source loading after 5 days postoperatively, ensuring minimal tension on the wound, and using free or rotational flap reconstruction (although such a complex reconstruction can be associated with additional complications) (32,33).
Chronic complications evaluated in patients who have received brachytherapy include bone fracture and neuropathy. The incidence of bone fractures in patients who have received brachytherapy for STS is 0% to 4.5% (27). There was no significant difference in fracture rates between cohorts in the MSKCC randomized trial (31). Neuropathy, although often evaluated as a late radiation complication, has not been definitively shown (23,31,34). Kubo et al reported on seven patients treated with HDR brachytherapy where the brachytherapy catheters were placed on neurovascular structures, and no peripheral neuropathy was reported and motor nerve conduction remained preserved (34).
SPECIAL SCENARIOS
Retroperitoneal Sarcoma
Although EBRT has proven to be beneficial in improving local control rates in extremity STS, its impact on sarcomas arising in the retroperitoneum has not been conclusively established and is the subject of an international phase III trial comparing preoperative EBRT plus surgery with surgery alone (ACOSOG Z9031). The proximity of the organs at risk (OAR) limits the dose that can be delivered through EBRT techniques. Although the impact of EBRT on retroperitoneal sarcoma (RPS) is unknown, there is additional interest in using even higher doses of EBRT to the perceived “at risk” margins of local failure. The potential benefit of dose escalation of RT to the “at risk” margins is controversial with some studies citing no proven benefit for dose escalation with either higher doses of EBRT, intraoperative radiation, or brachytherapy (35). Studies of intraoperative radiation therapy (IORT) have reported good local control with minimal side effects and led to further evaluation with brachytherapy (36–41). A trial from Princess Margaret Hospital evaluated preoperative EBRT followed by postoperative catheter-based brachytherapy and found significant toxicity with brachytherapy, with no improvement in disease control (42). Catheter-based brachytherapy is generally avoided for retroperitoneal sarcomas in high-volume sarcoma centers.
Table 12.2 Sarcoma monotherapy versus boost


Recurrent Disease in a Prior Radiated Field
The benefit of re-irradiation for recurrences in a previously radiated field is controversial. In support of re-irradiation, Catton et al noted 100% local control in their cohort of 10 patients who underwent surgery and radiation compared with 36% in the 11 patients who had surgery alone. The doses of radiation delivered ranged from 35 to 65 Gy (six brachytherapy, one brachytherapy plus EBRT, three EBRT). Wound healing complications were reported in 60% of cases (43). Pearlstone et al reported on 26 patients treated for a local recurrence with brachytherapy. The mean brachytherapy dose used at recurrence was 47.2 Gy. Local recurrence–free survival at 5 years was 52%, and DFS was 33%. The reoperation rate was 15%, and 50% of patients had planned tissue grafts (44). Nori et al published a series of 40 patients who underwent re-irradiation with brachytherapy with a median dose of 45 Gy. Local control at 5 years was 68% and treatment failures occurred in patients who had multiple recurrences. The complication rate was 12.5%. This report supported brachytherapy as salvage and advocated its use early in a local recurrence treatment paradigm (45). In contrast, Torres et al reported on 62 patients who underwent either surgery alone (generally as WLE) or surgery with brachytherapy (dose range: 45–64 Gy). The local control rates were not significantly improved in the cohort receiving brachytherapy. There was significant toxicity with an 80% reoperation rate in the cohort receiving radiation compared with 17% in the surgery-alone cohort (P < .001) (46).
Pediatrics
Rhabdomyosarcomas are the most common pediatric STS (47). Brachytherapy as part of the management of pediatric sarcomas can be used alone or in conjunction with EBRT. The dose falloff properties of brachytherapy techniques are especially valuable in the pediatric population as limiting dose to the surrounding normal tissue can have the dramatic effect of minimizing late toxicity and potentially decreasing the risk of secondary malignancies. Long-term follow-up of these patients is required to assess for toxicity (48). LDR and HDR techniques have been described in the literature with excellent local control rates (10,49–52). LDR temporary implant techniques can incorporate both iodine-125 (125I) and iridium-192 (192Ir) (52). The lower energy of 125I allows for decreased radiation exposure not only to adjacent tissues but also to family members (53). The use of brachytherapy in pediatric malignancies should be limited to cancer centers with a dedicated brachytherapy team with expertise in pediatric malignancies.
Skin
Superficial sarcomas, such as Kaposi sarcoma or angiosarcoma, can also be targeted with brachytherapy using superficial techniques (54,55). A recent study described the use of HDR brachytherapy to deliver 24 to 35 Gy in 4 to 6 fractions for Kaposi sarcoma lesions less than 2 cm. Complete clinical response to treatment with no evidence of local recurrence was observed in 16 of 16 cases over a median 41 month follow-up period (56). Superficial skin doses may be applied with either a commercial skin surface applicator or in-house surface molds. The dose per fraction and technique are impacted by the size of the lesion, as both Kaposi sarcoma and angiosarcoma are known to have a superficial spreading pattern with wide dissemination of lesions.
Permanent Implants
Permanent seed implants are routinely used in prostate brachytherapy and are being evaluated in the treatment of early breast and thoracic malignancies. Permanent seed implants have been described in situations when other techniques to deliver radiation to either gross disease or a positive margin were not feasible (57,58).
Recently, investigators from Brigham and Women’s Hospital and Dana-Farber Cancer Institute reported their experience with permanent implantable mesh brachytherapy in patients with deep cavity (retroperitoneum, pelvis, chest) STS (59). Brachytherapy, in the form of 125I seeds implanted in an absorbable polyglactan suture material embedded in a polyglactan mesh, was placed in the resection bed against the margins of concern. In this study, 83% of patients had recurrent sarcomas, and many had undergone EBRT at initial presentation. The “in-field” local recurrence rate was 26%, suggesting good local control in a cohort of largely recurrent sarcomas. However, the overall complication rate was quite high at 46%, with half of those being grade 3 or 4 complications. Thus, mesh brachytherapy must be used with caution after resection of deep cavity sarcomas, and only when other adjuvant options are limited.
INTERSTITIAL IMPLANT TECHNIQUE
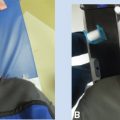
The most common technique for both LDR and HDR brachytherapy is an interstitial technique where the radiation oncologist and surgical oncologist, immediately following the surgical excision, place the interstitial catheters. The most common interstitial technique is a single plane of catheters either placed parallel to the surgical excision with blind-ended catheters or parallel to the incision with double leader catheters. Intraoperative placement of the catheters allows for direct visualization of the tumor bed, as well as the location of critical normal structures such as arteries, nerves, and bone. Consideration of the ease of loading of the radiation can also affect the placement of the catheters and drains (Figures 12.1A and B).
Defining the Clinical Target Volume
The clinical target volume (CTV) for brachytherapy is defined as the surgical resection bed plus a margin of 1 to 2 cm radially (including generally 2 cm cranio-caudally) (60,61). The size of the margin can be influenced by the size of the tumor, histology, and the completeness of the surgical excision. Intraoperative histologic evaluation of the margins helps one to define the CTV. The CTV for brachytherapy has not conventionally included the scar and drain sites.

Figure 12.1 (A) Blind-ended catheter being placed through metal trocar to lie parallel to the skin insertion. (B) A view of the catheters with buttons secured onto the tumor bed. The catheters are carefully affixed to the deep margin with interrupted chromic gut suture. The spacing reflects how the wound will come together at closure. Fewer catheters may be achievable as some of the target area will be folded over the catheters when the wound is closed. Manual approximation of the wound assists in this evaluation.
Intraoperative Catheter Placement
Once the CTV has been determined, the extent of the CTV should be marked with surgical staples. Critical normal structures (such as radiosensitive small intestine) may be protected through placement of a spacing material such as gel foam or tissue expanders provided that these materials do not compromise the CTV coverage. The radiation oncologist and surgeon can then discuss the best placement and orientation of the catheters based on the anatomy and coverage needs. Most implants are single planed, but in scenarios with gross residual tumor or concern about deeper involvement of muscle, multi-planed implants may be required.
The placement of the catheters may be parallel to the orientation of the wound or perpendicular. Advantages of parallel placement include fewer catheters, but when the volume is long or follows a curvature, perpendicular placement of the catheters may be preferred. Regardless of the orientation of the catheters, the principle of placement is the same. A metal hollow needle is introduced through the skin at a distance of 1 to 2 cm from the wound. The brachytherapy catheter is then advanced through the hollow needles onto the tumor bed. The distance between catheters ranges between 1 and 1.5 cm. Stabilization of the catheters to the tumor bed is essential. This can be achieved by using quickly dissolving absorbable sutures to tie down the catheters within the surgical bed. On the skin surface, the catheters are secured via anchoring devices such as spacers and buttons (Figure 12.2). Other novel techniques described use a Jackson–Pratt drain, whereby the catheters pass through the drain holes both within the wound and on the skin (62). The surgeon and radiation oncologist must also be cognizant of the resulting orientation of the catheters once the surgical wound is closed.
Postoperative Catheter Care
Postoperatively, the radiation oncologist should ensure that the catheters are in good alignment and are easily accessible. The recovery room staff and staff on the surgical ward should be educated to ensure that there is no damage to the catheter area. Any surgical drains placed intraoperatively should be left in situ until brachytherapy is complete and catheters are removed. The catheters should be kept clean and dry.

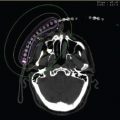
Figure 12.2 Buttons are used to secure the catheters to the skin. A variety of buttons are used. Nonmetallic buttons minimize scatter during CT. The buttons are stitched to the skin with small-gauge nylon sutures at the end of the case.
Planning and Dosimetry
Once the patient is through the acute postoperative recovery, he or she may be brought to the brachytherapy area for treatment simulation and planning. At this time, the catheters can be assessed for integrity. Brachytherapy catheters, in general, have an internal leader, which prevents the catheters from becoming kinked or stretched during the intraoperative procedure. These are carefully removed at the time of simulation and replaced with “dummy ribbons.” The dummy ribbons identify each individual catheter’s course through the CTV. It is practical to mark the external end of each single leader catheter with a marker to ensure that any physical measurements of length that are made at the time of simulation are unchanged at the time of treatment. Once all catheters have been prepared and numbered, CT simulation can occur. The patient should be scanned in the same position as the treatment. CT simulation allows for three-dimensional treatment planning, contouring of the CTV, and dose–volume histogram (DVH) analysis of the OAR. Conventionally, in brachytherapy treatment planning, there is no expansion to a planning target volume (PTV). D90 (dose to 90% of the CTV), V100 (percent of the CTV receiving 100% of the dose), V150 (percent of the CTV receiving 150% of the dose), and the skin dose should be recorded. There are no specific dose objectives or constraints related to D90, V100, or V150. There is generalized agreement to limit the dose to the incision to less than 100% of the isodose and limit the dose to skin to no more than two thirds of the prescription dose. Finally, source loading should be no closer than 0.5 cm from the skin surface.
Treatment Prescription and Delivery
The randomized study by MSKCC utilized the median peripheral dose rate (MPD) as the prescription point. This was defined as the minimum isodose line that continuously covered the CTV. This, in general, with a single plane implant with 1 to 1.5 cm spacing relates to approximately 5 mm depth into the tissue and approximates the 0.45 Gy/hr line. The prescription point for HDR and PDR brachytherapy has been extrapolated from these data, and for single-plane implants, it is usually defined as a distance of 5 mm from the implant. The dose is dependent on whether the treatment is brachytherapy alone or as a boost to EBRT. The ABS recommendations for LDR, HDR, and PDR dose prescriptions are found in Table 12.4.
Prior to delivering the radiation, it is important to evaluate the wound for postoperative complications, such as seroma, hematoma, wound breakdown, or infection, which may impact the radiation dosimetry or delay treatment. In general, it is recommended to wait until the fifth postoperative day to load the catheters (31). Source loading, however, has been described in the literature between postoperative day 2 to postoperative day 8 depending on the size of the wound and type of wound closure (60,61). Prior to each treatment, the catheters should be evaluated for movement and integrity. If there is any doubt about the catheter length, the patient should be rescanned with the dummy wires in place.
Catheter Removal
Once the treatments are completed, the catheters may be removed in a sterile fashion. The sutures to the stabilizing buttons or device are cut. In the case of blind-ended catheters, the catheter can be gently rotated or twisted to ensure that it is not adherent to either the skin or tissue within the closed wound and then gently pulled out. When double-ended catheters (in which both ends are protruding from the skin) are being removed, the principle is to not introduce an unsterile portion of the catheter (ie, a portion of the catheter that was already external to the skin) back into the surgical bed. Therefore, after the skin sutures are cut, gentle pressure is applied on the skin over the catheters to expose part of the catheter that had been subcutaneous, and this is cut enabling the short external end to be removed. The remainder of the catheter can then be pulled through the surgical bed.
Table 12.4 Common doses used in sarcoma brachytherapy

Superficial Mold Technique
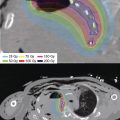
Surface sarcomas such as angiosarcoma and Kaposi sarcoma can be treated with a superficial mold technique. In these situations, the CTV is usually delineated with wires, and a contoured applicator or mold is created to affix to the treatment area. The patient then undergoes a planning CT with the applicator affixed in the treatment position. The CTV can be determined by the wiring prior to the CT (Figures 12.3A and B).
CONCLUSION
Brachytherapy is an effective mechanism for delivery of RT to improve local control after resection of localized STS. However, efficacy has only been demonstrated in a randomized trial for extremity STS. Some of the toxicity associated with the addition of adjuvant brachytherapy can be offset by meticulous timing, such as avoiding loading catheters until postoperative day 5.
It is not known whether brachytherapy truly adds any benefit when coupled with modern approaches to EBRT, such as IMRT. Such a question could be addressed in a prospective trial. Both catheter-based brachytherapy and mesh-based permanent implantable brachytherapy for abdominal, pelvic, retroperitoneal, and other deep cavity STS are associated with significant morbidity and, in the former case, mortality. Therefore, indications for brachytherapy may be limited, in those scenarios, to situations in which there are really no good options for EBRT for expected close or positive margins and only to experienced, high-volume sarcoma centers.


Figure 12.3 (A) Superficial angiosarcoma of the scalp with the CTV marked by the CT wire prior to CT planning. (B) A superficial applicator is created to cover the area defined as the CTV. (C) CT planning scan showing the applicator and dose distribution. (D) Three-week follow-up showing resolution of moist desquamation. CTV, clinical target volume.