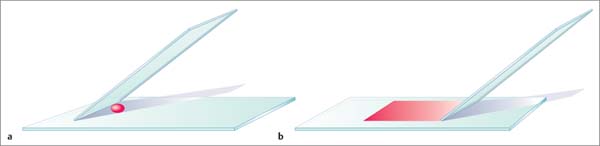
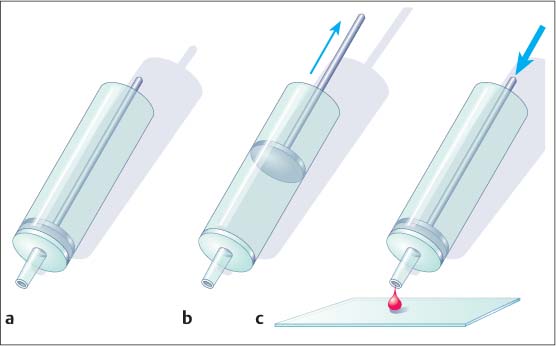
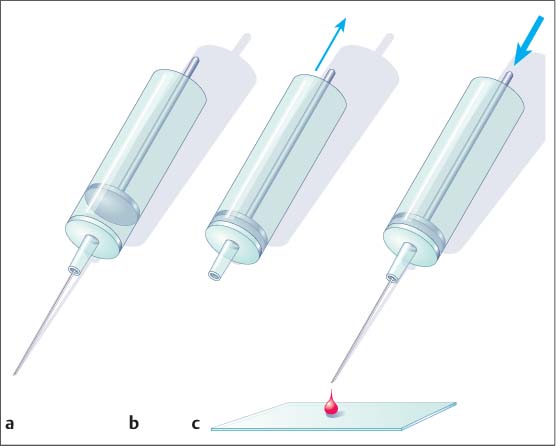
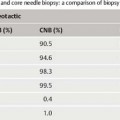
8 Specimen Processing for Pathologic Diagnosis In the diagnosis of breast anomalies, cytologic smear examinations are usually performed when a bloody nipple discharge is present. Despite the fact that a bloody nipple discharge is caused by intraductal papillomas and other benign changes in approx. 95% of cases, exfoliative cytology is performed to exclude the presence of malignant cells in the nipple discharge. A negative cytologic result, however, does not reliably exclude malignancy. To attain secretion for a cytologic smear, the breast should be gently massaged from the periphery toward the nipple. By slightly squeezing behind the nipple, the subareolar secretion accumulation can then be expressed and collected on one end of a glass slide. A second specimen slide is then used to smear the secretion drop across the greater length of the first slide for cytologic examination (Fig. 8.1). The smear is most commonly air-dried and then labeled and prepared for transportation to the cytopathologist. The indication for the performance of a fine needle aspiration biopsy (FNAB) is limited to the aspiration of symptomatic or complicated cysts, the diagnostic work-up of palpable lumps without correlating imaging abnormalities, and the diagnostic work-up of suspicious axillary lymph nodes. Fluids. The syringe in which aspirated fluids (e. g., from a cyst) are collected is closed with a stopper and labeled with the patient data before being sent to cytopathology. The fluid is then centrifuged and the precipitate smeared onto a specimen slide, air-dried, and stained (Giemsa stain) for microscopic examination. Solid lesions. FNAB of a solid lesion (e. g., lymph node) is performed by rapidly passing the needle back and forth in various directions, 1 to 2 cm through the lesion, while applying a vacuum (negative pressure) to obtain cellular material. After the pressure in the syringe has been allowed to equalize, the needle is removed from the breast. The contents of the syringe and needle are then carefully expressed onto a glass slide. For this, the syringe is first disconnected from the needle and filled with air by drawing back the plunger. The Luer-slip is then held closely over the glass slide and the material expressed by applying positive pressure (Fig. 8.2). This procedure is repeated after reconnecting the needle to the syringe after the plunger has been drawn back (Fig. 8.3). Tips and Tricks Occasionally, the aspirated material can be scanty and located in the syringe, making it difficult to retrieve. In such cases, it is sometimes possible to use a long needle to salvage the sample for the cytologic smear. Alternatively, saline solution can be aspirated into the syringe through the puncture needle, retrieving the cellular material herein. The syringe is closed with a stopper and then centrifuged at the laboratory. The precipitate is then used to prepare the cytologic smear. Viscous aspirate. Cytologic specimens of viscous aspirate are best obtained by applying flat pressure with the spreading slide on the slide with the sample drop and then distributing it by moving the slide sideways (Fig. 8.4). Fig. 8.1 a, b Smearing fluid aspirate. Fig. 8.2a–c Removing aspirate from a syringe. a Syringe with aspirate. b The plunger is drawn back slowly. c The Luer-slip is held closely over the glass slide and the material expressed by rapidly applying positive pressure. Fig. 8.3a–c Removing aspirate from the puncture needle.
Cytologic Smears
Aspiration Cytology
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree