SPINAL ACCESSORY NEUROPATHY
KEY POINTS
- Computed tomography including computed tomographic angiography is the primary tool for looking for a structural cause of a cranial neuropathy that is likely to involve the skull base or more distal course of the nerve in question.
- Magnetic resonance imaging should be used if the pathology likely localizes to the brain stem or cisternal segment of the nerve(s) in question.
- Magnetic resonance imaging and magnetic resonance angiography cannot be trusted to confidently exclude all conditions that may lead to these neuropathies.
- The causative pathology may be of the end organ or other structures innervated by the nerve rather than the nerve itself.
- Diagnostic imaging, even performed optimally, fails to find a reason for these neuropathies in many patients.
INTRODUCTION
Clinical Presentation
The spinal accessory, glossopharyngeal, vagus, and hypoglossal nerves and cervical sympathetic plexus may be considered as a unit as well as individually. Although one of these may dominate the clinical presentation, more than one will often be involved following careful clinical testing; multiple nerve involvement is often the case when a spinal accessory neuropathy is present. When more than one nerve is affected, and possibly associated with other symptoms or neurologic deficits, localization may be much easier and a very “directed” and concise imaging evaluation is possible. However, some situations require a comprehensive imaging study of nearly the entire head and neck region and sometimes the upper chest. For instance, cranial nerve (CN) X and cervical sympathetics have a long course between the brain and skull and the chest, and CNs IX and X have important pathways of referred pain. Such considerations and combinations of clinical findings may affect the imaging approach to identify a source of a patient’s presenting complaints and deficits. This clinically responsive tactical approach should become apparent later in this chapter as well as in Chapters 137, 138, 139, and 141.
The spinal accessory nerve has a long course between the brain and skull and the low neck and chest. This may affect the imaging approach to a patient’s presenting complaints and deficits, as will become apparent later in this chapter; however, for practical purposes, it is most typically affected by the pathology of the skull base and upper neck.
Sometimes, lesions outside the expected course of the nerves or those involving end points of innervation will be the source of symptoms—for example, a tongue base carcinoma causing ear pain that is referred along the glossopharyngeal nerve. It is critical to understand all of the neurologic deficits and complaints that might arise from these nerves to enable effective imaging and interpretation of the images.
Isolated spinal accessory nerve neuropathy short of that related to surgical sacrifice is very uncommon. Pathologic processes usually involve the IX, X, XI complex. Isolated spinal accessory nerve pathology will cause dysfunction of the sternocleidomastoid and/or trapezius muscle and eventually some degree of atrophy.
Primary muscle problems resulting in functional loss of muscle action rarely come to imaging since the cause of the problem will be known clinically, such as end-stage atrophy and fibrosis due to infantile myositis and related torticollis.
Clinical presentations that may involve CN XI are outlined as follows:
Lesions Within the Skull:
- Brain stem and extra-axial lesions in the low cerebellopontine angle and medullary cisterns may present as lower CN deficits. Intra-axial lesions will almost always be accompanied by some sensory or motor symptoms and related physical findings. This is virtually never the site of origin for an isolated spinal accessory neuropathy.
Jugular Fossa Syndromes
- Several French eponyms have been applied to clinical findings when three, four, or five (if the sympathetics are included) members of this group of nerves are involved. These include Vernet syndrome (IX–XI), Collet-Sicard syndrome (IX–XII), and Villaret syndrome (IX–XII and the sympathetic plexus).
- French eponyms are mentioned because they are occasionally presented as an indication for the imaging procedure; however, it is more useful to describe the specific nerves at risk. If three of the five are involved, then the lesion will be at or near the skull base. The lesion will usually be somewhere between the medullary cistern–jugular fossa region and the posterior belly of the digastric muscle. If just two are involved, then the lesion could lie in this relatively confined space in and around the skull base but a more comprehensive imaging evaluation often becomes necessary.
APPLIED ANATOMY
Cranial Nerve Nucleus and Cisternal Segment
The accessory nerve originates in the medulla oblongata and in the cervical spinal cord (Fig. 139.1). The cranial component of CN XI and CNs IX and X share origins in the nuclei of the medulla. Motor fibers of all three nerves originate in the nucleus ambiguus dorsal to the inferior olivary nucleus. The fibers course in the tractus solitarius and end in the nucleus solitarius. Once they leave the lateral medulla, all roots traverse the basal cistern, sometimes conjoined. The spinal component originates at the level of C1 to C4 in the lateral region of the anterior horn. Fibers leave the spinal cord near the posterolateral sulcus to form a spinal root that ascends through the spinal canal and foramen magnum to join the cranial roots.


FIGURE 139.1. Anatomy of the spinal accessory nerve. A–D: Anatomic drawings of the brain stem nuclei (A), cisternal segment (B), skull base segment coursing through the jugular foramen and partly joining the vagus nerve (C), and the peripheral segment of cranial nerve (CN) XI. E–H: Anatomic sections of CN XI. The nerve originates in the cervical spinal cord (not shown) and medulla oblongata (N). Together with CNs IX and X, it courses through the medullary cistern to the pars vascularis of the jugular foramen. Cranial fibers unite with the vagus nerve at this level to innervate the soft palate, pharynx, and larynx. The spinal fibers continue within the carotid sheath, between the internal carotid artery and jugular vein, and course inferior to the digastric muscle to terminate in the trapezius muscle. (N, brain stem nucleus; c, carotid artery; j, jugular vein; SYM, sympathetic plexus; DM, digastric muscle.)
This is virtually never the site of origin for an isolated spinal accessory neuropathy.
Foraminal/Skull Base Segment
The more medial and smaller of the two jugular fossa compartments known as the pars nervosa contains the terminus of the inferior petrosal sinus and the glossopharyngeal nerve (CN IX) (Figs. 137.2 and 139.1). The more lateral of the two known as the pars vascularis is larger and contains the jugular bulb, vagus nerve (CN X), and spinal accessory nerve (CN XI) (Fig. 104.10A–C). Fibers from the cranial root will join the inferior ganglion of the vagus nerve (internal branch, accessory to the vagus). Fibers from the spinal root form the external branch, commonly referred to as spinal accessory nerve. This is virtually never the site of origin for an isolated spinal accessory neuropathy.
Peripheral Segment and Distal Innervation
The spinal accessory nerve follows the carotid sheath to the level of the posterior belly of the digastric muscle, where it diverges laterally and crosses either in front or in back of the jugular vein, eventually giving off branches to the sternocleidomastoid and trapezius muscles as it courses distally in the posterior triangle (Fig. 139.1).
Innervation End Organ
The internal branch of CN XI unites with the vagus nerve and supplies motor innervation to the muscles of the pharynx, larynx, and soft palate (Fig. 139.1). The external branch supplies motor innervation to the sternocleidomastoid and trapezius muscle.
IMAGING APPROACH
Techniques and Relevant Aspects
General imaging parameters used in cranial and cervical sympathetic neuropathies often require a combination of high-detail images from the posterior fossa to the hyoid bone and more of a survey approach from the angle of the mandible to the low neck. Overall, multidetector computed tomography (MDCT) is well suited to accomplishing all of these tasks very efficiently, and magnetic resonance imaging (MRI) can be focused on a specific area for problem solving when required.
For the patients with spinal accessory neuropathy, a complete contrast-enhanced computed tomography (CT) study will usually include temporal bone and posterior skull base reconstructions with the same technique used to study primary temporal bone problems and high-detail images from the posterior fossa to the mandibular angle. A survey of the neck may be required.
Magnetic resonance (MR) may be used for the investigation of a spinal accessory neuropathy if the lesion is likely intracranial; however, MR is more likely to be degraded in the pharynx below the hard palate due to motion artifacts and does not definitively exclude smaller skull base lesions.
Specific protocols for CT and MR studies for investigating a spinal accessory neuropathy appear in Appendixes A and B.
Diagnostic catheter angiography may be necessary in some instances to confirm the diagnosis of a paraganglioma if it is equivocal on imaging. Until newer MDCT techniques such as the 320 MDCT units released in 2008 that are capable of assessing flow dynamics and detailed angioarchitecture become generally available, diagnostic catheter techniques will remain necessary to assess these parameters, often as a prelude to endovascular interventions that are usually an adjunct to surgical removal.
Radionuclide studies are used sparingly in cases likely to be due to skull base osteomyelitis. Fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) should have a minor adjunctive role in evaluating diseases that lead to these neuropathies.
Pros and Cons
MRI and magnetic resonance angiography (MRA) are not well suited to definitive evaluation of the region of the jugular fossa and posterior skull base. These imaging studies cannot confidently exclude causative pathology as being the main limitation; however, even obvious pathology may not be as definitively evaluated as with CT and computed tomographic angiography.
These limitations are essentially related to the inability of MRI to provide definitive bone information and because of the variability in the area of main interest around the petromastoid junction caused by the common occurrence of bone, air, and fat interfaces. This limitation of MRI and MRA is further complicated by flow-related artifacts that are often exacerbated by the use of contrast (Chapter 104).
On the other hand, if the lesion is likely to be intracranial—either purely intra-axial or caused by leptomeningeal pathology—the use of MRI for the detection of those processes is clearly superior to CT. However, these intracranial sites are much less frequently the origin of disease in neuropathies that present without somatic sensory or motor deficits. MRI may be used first when the pathology is likely to be meningeal or intra-axial, although even in such cases well-done initial CT would produce at least suspicious findings that would lead to MR in any case.
PATHOPHYSIOLOGY AND PATTERNS OF DISEASE AS SEEN ON MAGNETIC RESONANCE IMAGING AND COMPUTED TOMOGRAPHY
Introduction
The specific pathologies that involve the CNs are variable but can be grouped by three broad mechanisms of disease, including primary neurogenic tumors, pathology that causes a particular point of compressive neuropathy, and infiltrating neuropathies. It is essential to first determine the specific localization of the offending pathology; this is logically and perhaps best approached by a systematic evaluation of the nerve from its brain stem origin to its end organ(s) that generate the signs and symptoms of disease. Following that, a morphologic evaluation of the disease process will almost always lead to a specific diagnosis or very short list of possibilities. Spinal accessory neuropathy is almost always associated with other CN deficits.

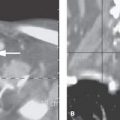
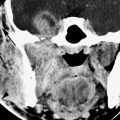
FIGURE 139.2. Intra-axial disease causing lower cranial nerve deficits. Progressive demyelinating disease involving the left brain stem causing cranial nerve XI and XII neuropathies clinically.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree