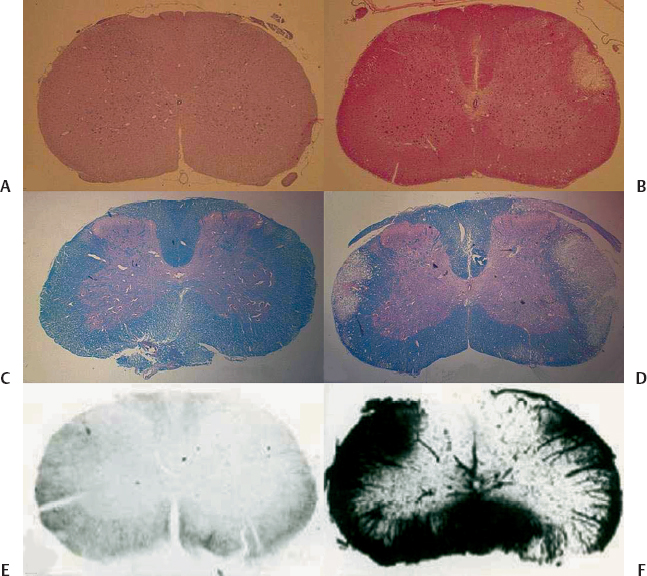
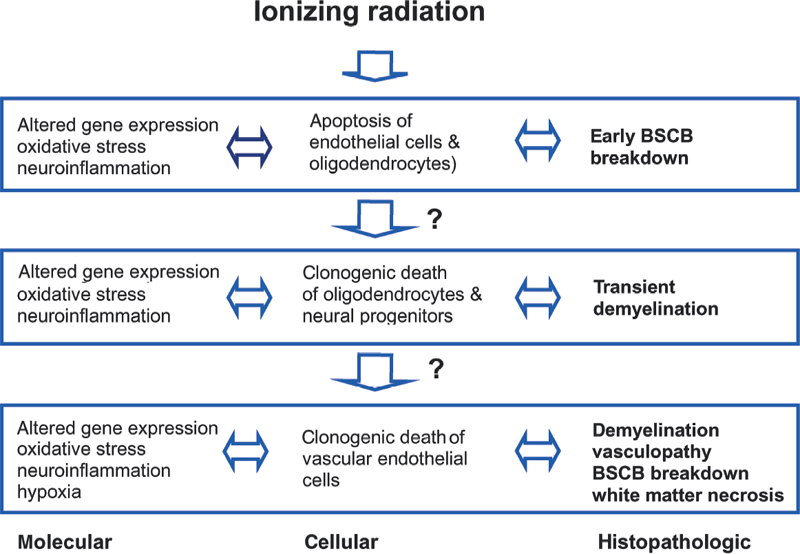
2 Central nervous system (CNS) radiation injury is a major toxicity in radiation therapy (XRT) for tumors located within or near the brain and spinal cord. White matter necrosis, demyelination, and vascular damage (including increased vascular permeability) are prominent histopathologic findings following XRT to the CNS. Treatment-related sequelae such as focal neurological deficits, neurocognitive dysfunction, and myelopathy are of such concern that they severely limit the clinician’s ability to deliver curative doses of XRT. Radiation injury to the CNS is manifested as three distinct clinical entities.1 During a course of XRT, patients often experience fatigue and an exacerbation of preexisting neurologic symptoms and signs. However, death from brain herniation, especially after a large single dose as delivered during stereotactic radiosurgery, has been reported.2 These acute CNS radiation injuries (early effects) are generally considered to be secondary to edema and disruption of the blood-brain barrier (BBB).3,4 A self-limiting delayed reaction known as Lhermitte syndrome is well recognized after XRT to the spinal cord. It occurs after a latent period of 2 to 4 months and is characterized by paresthesia in the back and extremities upon neck flexion. After cranial XRT, a corresponding syndrome characterized by somnolence has been described (somnolence syndrome).5 These syndromes typically last for a few months followed by complete clinical recovery. Transient demyelination is believed to be the underlying mechanism of these early delayed reactions (subacute effects).6 There is, however, no correlation of Lhermitte syndrome with permanent radiation myelopathy. Late effects are typically irreversible and most devastating, and thus are the most clinically important. Radiation myelopathy is one of the most distressing late complications of radiotherapy. It generally occurs after a latent time of 12 to 14 months, but it has been reported 3 to 4 years after XRT.7,8 Clinical symptoms and signs of radiation myelopathy vary depending on the level of injury; they can occur in various combinations of motor and sensory deficits and at different rates of progression. Moreover, myelopathy can be fatal if the damage occurs at the upper cervical level.9 Initial signs of radiation-induced myelopathy may be non-specific and often include a diminished temperature sensation, diminished sense of proprioception, muscle weakness (typically beginning in the legs), and clumsiness. Changes in gait, incontinence, Brown-Séquard syndrome, spasticity, paresis, plegia, hyperreflexia, and the Babinski sign are examples of more characteristic symptoms and signs that develop as the injury manifests. The spinal cord is an elongated cylindrical structure and an extension of the brain. In the adult mammal, the spinal cord extends from the superior border of the atlas (foramen magnum) to the lumbar vertebrae. In the human adult, the spinal cord typically ends at the junction between the first and second lumbar vertebrae (L1-L2) and is typically 45 cm in length.10 The spinal cord is bathed in cerebrospinal fluid (CSF) and enclosed by the dura, arachnoid, and pia mater, which are separated by the subdural and subarachnoid spaces. A spinal cord segment provides the attachment of the rootlets of a pair of spinal nerves. From both sides of a segment of cord, dorsal and ventral roots traverse the dura and unite close to their intervertebral foramina to form mixed spinal nerves.10,11 Nerve roots of the lumbar and sacral segments of the cord form a bundle of roots (known as the cauda equina) as they descend to their respective intervertebral foramina. In total there are 31 pairs of spinal nerves (i.e., segments).10,11 The spinal cord lies inside the spinal column, which is made up of 33 vertebrae in the human. Five vertebrae are fused together to form the sacrum, and four small vertebrae are fused together to form the coccyx. The spine is typically divided into four sections (excluding the coccyx): the cervical (C1-C7), thoracic (T1-T12), lumbar (L1-L5), and sacral (S1-S5) vertebrae.10,11 Between the vertebral bodies (except C1 and C2) are disks that serve as the supporting structure for the spine. Ligaments attached to the vertebrae also serve as supporting structures. The transverse diameter of the cord gradually tapers craniocaudally except at the level of two enlargements. The cervical enlargement (C3-T2) is the source of large spinal nerves innervating the upper limbs, and the lumbar enlargement (L1-S3) is the source of large spinal nerves supplying the lower limbs.10,11 On transverse section of the cord, the basic structural organization consists of central gray matter and outer white matter. Ascending tracts of sensory fibers and descending motor tracts are the main components of the white matter that are organized into funiculi (white columns) and grouped into dorsal, lateral, and ventral funiculi. Gray matter contains the neuronal cell bodies, and on transverse section it resembles the letter H with ventral and dorsal horns. The more prominent ventral horns contain cell bodies of the large lower motor neurons, and the dorsal horns contain the cell bodies of second-order sensory neurons involved in pain and temperature sensation. Details of specific cell columns and tracts are not the focus of this chapter and can be obtained from neuroanatomy textbooks. The major cell types in the spinal cord, similar to those in the brain, are neurons, glia, and vascular endothelial cells. Neurons are of neuroectodermal origin and, despite great variation in size and shape, consist of a cell body (containing the nucleus and surrounding cytoplasm known as the perikaryon), a short number of dendrites, and a long, single, straight axon. The receptive parts of the neuron are the dendrites, and the axon transmits the impulse to its terminal branches. Glial cells are also of neuroectodermal origin and are generally divided into oligodendrocytes, astrocytes, and microglia. Oligodendrocytes are responsible for myelination of axons and dendrites in the CNS.12 The process of myelination allows propagation of nerve impulses or action potentials. Myelin sheaths are formed by the wrapping of oligodendrocyte cytoplasmic processes around the axon and are interrupted by the nodes of Ranvier, which serve to enhance propagation of action potentials. One oligodendrocyte can myelinate segments of several adjacent axons via its processes. Astrocytes are irregularly shaped glial cells with a large number of branched processes. Many of these processes end in terminal expansions upon the basement membrane of capillaries known as perivascular feet. Astrocytes participate in neuronal growth and development, as well as in the transmission of the neuronal signals.13 They also play a key role in the formation and maintenance of the BBB and, in the spinal cord, the blood-spinal cord barrier (BSCB).14 Microglia are small cells that, upon activation in response to tissue damage, transform into phagocytic cells. They are thought to be part of the monocyte-macrophage defense system in the brain and spinal cord.15 Vascular endothelial cells in the spinal cord are responsible for the BSCB. Similar to the BBB, the BSCB severely restricts the passage into the spinal cord of most proteins, hydrophilic molecules, and ions in the circulation. In addition to the specialized endothelium of the spinal cord, other components of BSCB include the basement membrane, astrocytes, and pericytes in immediate proximity. The unusual impermeability of CNS capillaries is attributed to tight junctions between endothelial cells and a paucity of endothelial vesicular transport. These features are the basis for the characteristic resistance to para- and transcellular transport, respectively.16,17 It is now recognized that neural progenitors and neural stem cells persist in the adult CNS.18,19 Although these cells are generally believed to reside in certain areas of the brain, they can be isolated from the adult spinal cord.19 The role of these cells in the radiation response of the CNS, including the spinal cord, is beginning to emerge, as discussed later. Recent studies have highlighted the importance of interactions of endothelial cells, glia neurons, and neural progenitors in the normal and diseased CNS.20,21 An understanding of how these interactions are disturbed and disrupted in spinal cord radiation injury could lead to the development of novel neuroprotective strategies. XRT for patient therapy is ionizing, meaning that its photons, upon interaction with matter, contain sufficient energy to release an electron orbiting around the nucleus of an atom or molecule. XRT can cause any damage to any molecule in a cell, but damage to deoxyribonucleic acid (DNA) is most crucial in causing cell lethality. Damage to DNA can occur directly through the free electrons themselves or indirectly through free radicals generated from these electrons.22 Cellular damage following XRT may lead to cell-cycle arrest, mitosis-linked death, or apoptotic cell death.23 The probability of cell survival after a single dose of XRT is a function of absorbed dose and is measured as energy per unit mass of tissue in gray units (Gy). In cells cultured in vitro following XRT, the capability of a single cell to grow and form a large colony is typically used as a measure of reproductive integrity. The formation of these colonies provides the basis for quantitative assays that can be used to describe the survival of cells after XRT. Whereas differentiated cells such as neurons, astrocytes, and oligodendrocytes in the CNS do not form colonies, neural stem cells and progenitors can be cultured in vitro and form colonies under specific growth conditions. These assays have been used to characterize the clonogenic survival of neural progenitors isolated from the spinal cord after in vivo XRT.24,25 Many different mathematical models have been used to describe cell survival after XRT. One that is widely used in the clinic is the linear-quadratic model,26 and it can be used to describe the fractionation sensitivity of tissues after XRT.27,28 Late-responding normal tissues such as the spinal cord have low values of the alpha to beta (α/β) coefficient based on the linear-quadratic model. This is indicative of tissues where significant sparing can be achieved with fractionation. Values of α/β for the spinal cord ranged from 1.5 to 3.0 Gy.29,30 The concepts of cell survival curves and fractionation are discussed in Chapter 1. It should be pointed out these mathematical models, including the linear-quadratic model, are likely to be overly simplistic and flawed, given what is currently known about the molecular responses of cells and tissues after XRT. A functional assay that has been used extensively to assess the radiation response of the spinal cord is paralysis.30,31 The assumption is that the defined level of functional deficit(paralysis) corresponds to a decrease in the number of surviving target cells in the spinal cord. In rats, the ED50 (the estimated isoeffective dose for induction of paralysis in 50% of animals) for forelimb paralysis due to white matter necrosis was 19 Gy following single doses to the cervical spinal cord and went up to 87 Gy for 40 daily fractions.30 Therefore, the total dose to cause necrosis of the spinal cord is much lower for a single dose of radiosurgery as compared with conventional fractionation. When it comes to sparing of the spinal cord, it must be emphasized that there are no radiobiological advantages of single high doses as compared with fractionated regimens for malignant tumors. With a single high-dose treatment for a given rate of tumor cure, or control, the risk of myelopathy is much greater as compared with fractionated regimens. Therefore, a narrower therapeutic ratio must be respected, as the risk of late effects increases with increasing dose per fraction. Technology has evolved such that single high-dose treatments can be delivered safely and precisely with the aim of equivalent or even better tumor control, as compared with fractionated regimens, while minimizing dose delivered to organs at risk. The net result is a shorter overall patient treatment time, and therefore improved patient convenience while maintaining a sufficient therapeutic ratio. Many proteins and complex signaling cascades are involved in the general molecular response to XRT.22,23 These molecular events are described in several textbooks on radiobiology.22,23,26 The cellular and molecular details to follow in this chapter will be specific to spinal cord radiation. As spinal cord injury (myelopathy) is objectively detectable by the onset of paralysis, radiation-induced cord injury has been studied in greater detail in animal models than has brain injury. Much of what is known about the radiation responses of the CNS has been based on radiobiological studies of the spinal cord. Most studies of spinal cord radiation responses were performed in rodent species, particularly in rats, using single doses. These studies are thus particularly relevant to spine radiosurgery. In rat spinal cord, forelimb paralysis is observed at 4 to 5 months after single doses exceeding 20 Gy.30 In these animals, the most important and predominant histopathologic change is necrosis confined to white matter (Fig. 2.1A,B). Figure 2.1 (A,B) Low-power views of transverse sections of control (A) and irradiated (B) cervical rat spinal cord on routine hematoxylin and eosin staining. (B) In animals that developed forelimb paralysis at 20 weeks, there is evidence for necrosis confined to white matter after a myelopathic dose of 25 Gy. (C,D) Nonirradiated spinal cord shows no evidence of demyelination upon luxol blue staining for myelin (C), whereas demyelination is evident in lateral white matter at 20 weeks after 22 Gy (D). (E) Using horseradish peroxidase as a vascular tracer, control nonirradiated spinal cord shows no extravasation of the tracer. (F) The transverse section of the cervical spinal cord at 17 weeks after 25 Gy shows reaction product of horseradish peroxidase in almost all of the white matter. Disruption of the blood-spinal cord barrier invariably precedes histologic evidence of demyelination and necrosis. Histologically, these changes consist of randomly distributed areas of cell loss, necrotic debris, swollen axons, and focal to extensive demyelination and necrosis (Fig. 2.1C,D). A scant mononuclear infiltrate is occasionally seen.30 There are no specific vascular changes observed for radiation myelopathy. In rat spinal cord, the vascular changes are generally not prominent, although foci of fibrinoid degeneration of vascular walls are sometimes observed. In other mammalian species, vascular changes that have been described include telangiectasia, perivascular fibrosis and inflammation, hyaline degeneration and thickening, edema and fibrin exudation, stagnation and leakage of erythrocytes, and thrombosis with vessel obliteration and hemorrhage.32,33 Perhaps the most consistent vascular abnormality is the disruption of the BSCB. In rat spinal cord, gross histopathologic changes were invariably preceded by a generalized disruption in vascular permeability in white matter (Fig. 2.1E,F).34 Studies that described histopathological changes at lower doses, or doses below the threshold of paralysis, have not been reported as often. Similarly, few studies have tracked the histopathologic changes during the latent time prior to the onset of paralysis. In rat spinal cord following doses of only 5 to 10 Gy, paranodal demyelination, nodal widening, and wallerian-type degeneration of fibers in white matter has been described as early as 2 weeks postradiation.35,36 However, in the 5 to 10 Gy dose range, remyelination was observed at 3 months post-XRT. This observation has led investigators to conclude that early delayed effects (Lhermitte syndrome) are related to demyelination that could occur at doses below the threshold for tissue necrosis. In rhesus monkeys that underwent XRT to the cord but did not develop myelopathy by 2 years, the lesions consisted of single or multiple small mineralized foci in nonmotor tracts, astrocytes that were increased in size and often adjacent to foci of malacia, and microglial cells surrounding flecks of mineral.37 In addition, vasculopathy of hyaline degeneration was frequently observed as both an independent process and in close association with lesions of the neurophil (brain tissue that lies between cell bodies). Histopathologic description of human myelopathy is typically based on case reports or small series where autopsy materials were available. In an analysis of these reports, there were lesions that involved only white matter parenchyma with minor vascular changes, lesions that were mainly vascular, and lesions that demonstrated characteristics of both white matter parenchyma and vascular damage.38 Human studies are limited due to many unknown patient, tumor, treatment, and other confounding factors that may influence the development of damage. In addition, the damage reported typically represented patients with the most severe damage, such that histologic or autopsy materials were available for examination. For decades, largely based on extrapolation of histopathologic changes described above, the debate on the pathogenesis of spinal cord myelopathy after ionizing radiation was focused on the role of the oligodendrocyte versus the vascular endothelial cell. The support for the “glial hypothesis” stems from the observation of demyelination and lesions confined to white matter, whereas the “vascular hypothesis” stems from the observation of disruption of the BSCB that precedes or is associated with white matter lesions and other vascular changes observed. With recent advances in neurobiology, it is now recognized that spinal cord injury is unlikely to be related only to clonogenic cell death of one or two target cell types. Whereas at the clinical and histopathologic level, there is a latent period of months to years prior to the onset of myelopathy, at the cellular and molecular level, there is no evidence of a latent time. The development of spinal cord damage is currently best viewed as a damage continuum culminating in tissue necrosis with clinical myelopathy. In terms of mode of cell death in the CNS after XRT, certain glial, neuronal, and endothelial cells in the CNS also undergo apoptosis within hours after XRT. However, the clinical relevance of this mode of cell death remains unclear.4,39–43 Furthermore, there is also a component of secondary injury and cell death that may be mediated by microenvironmental alterations such as hypoxia/ischemia and neuroinflammation.44–48 Figure 2.2 illustrates a model of the molecular and cellular responses in the irradiated spinal cord. Insight into potentially reversible components of damage may offer neuroprotective avenues against radiation injury. Ionizing radiation produces a variety of DNA and other cellular lesions that elicit a stress response. Altered gene profiles represent one characteristic feature of this response. In the rodent CNS, increased expression of proinflammatory and other genes has been demonstrated within hours following XRT.49–52 These include genes of transcription factors such as nuclear factor-kappa B (NF-κB), cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), basic fibroblast growth factor (bFGF), adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), and selectins. NF-κB is important in the regulation of cytokine expression, including expression of TNF-α and IL-1β, both of which have been implicated in the development of demyelination.53 The expression of TNF-a is associated with edema observed after ischemic and hypoxic injury,54 and is also a key regulator of ICAM-1. ICAM-1 is associated with BBB disruption in a variety of injuries,55 and increased ICAM-1 expression has been observed in rat spinal cord within 24 hours after XRT.56–58 Increased neurologic abnormalities and extensive demyelination were observed in TNFRp75-deficient mice after cranial irradiation as compared with TNFRp55-deficient and control mice. These findings implicate a role for TNF-a in radiation-induced demyelination.59 Similar to other CNS injury models, a state of oxidative stress is associated with an increase in reactive oxygen species (ROS). Levels of malonaldehyde (MDA), an end product of lipid peroxidation, were found elevated in the mouse hippocampus at 1 week after a dose of 10 Gy, and cells immunoreactive for MDA were observed in the dentate gyrus at 24 hours.60 It has been suggested that the ROS responses may be tied to p53-dependent regulation of cell cycle control and stress-activated pathways. Oxidative stress and ROS are associated with activation of heme oxygenase 1 (Hmox 1), a stress protein,61 and a dose-dependent increase in Hmox1 gene expression has been observed in rat spinal cord after XRT.62 Oxidative stress and increases in ROS are likely to interact with radiation-induced altered gene profiles and to participate in responses to XRT of the CNS. Figure 2.2 A model of the molecular and cellular responses of the irradiated spinal cord. Whereas at the histopathologic level injury to the spinal cord after irradiation may be distinct entities, at the cellular and molecular level, the response is best viewed as a continuous and interacting process in which altered gene expression, neuroinflammation, and oxidative stress all contribute to cell kill induced by irradiation. BSCB, blood-spinal cord barrier. Recently, microarrays have allowed for the study of global gene expression in rodent CNS after XRT. These studies showed very complex patterns of gene expression profiles in mouse brain after XRT, and these expression profiles appeared to be dose- and time-dependent after cranial XRT.63,64 When expression profiles in the irradiated mouse kidney were compared with those in the irradiated brain, striking differences were observed. These observations are consistent with the notion that cellular and tissue response to XRT is complex and critically dependent on cell and tissue types.65 Microarray analysis may allow the identification of novel genes that may play a role in CNS radiation injury. It is now recognized that clonogenic cell death is not the only mode of cell death in the CNS after XRT.4,39–43 Following single doses, an apoptotic response occurs within hours in rodent spinal cord and is not observed by 24 hours. The apoptotic cells that have been best characterized in the spinal cord are oligodendrocytes.39,40,66 Some of the apoptotic cells have the phenotype of oligodendroglial progenitors.67 In the irradiated spinal cord, following an apoptotic response of oligodendrocytes peaking at approximately 8 hours, a reduction of oligodendrocytes was observed at 24 hours.39,66 After XRT of the rat cervical spinal cord with 15 or 23 Gy, the oligodendroglial progenitor cell (OPC) pool was reduced to < 0.1% of its normal population at 2 to 4 weeks. This was followed by a dose-dependent recovery reaching a maximum of 40 to 80% of control values at 3 months.68 This recovery was preceded by proliferation of OPCs. Early proliferation of OPCs, however, was not impaired in the irradiated spinal cord of p53-knockout mice. Given the dependence of radiation-induced apoptosis of oligodendroglial cells on p53, this suggests that the early proliferation of oligodendroglial progenitors observed may not be linked directly to apoptosis of oligodendrocytes.67 In rat spinal cord, several early events such as oligodendroglial apoptosis, reduced oligodendroglial density, and changes in oligodendroglial gene expression were observed after a myelopathic dose of 22 Gy. Similar changes occurred after a much lower dose of 8 Gy. In addition, only after a myelopathic dose was there a secondary decline of OPCs, with subsequent demyelination, observed between 4 and 5 months. These findings suggest that these early cellular and molecular events affecting the oligodendroglial populations are unlikely to be directly associated with late demyelination observed after XRT.66 OPCs are bipotential glial (O-2A) progenitors that can also differentiate into astrocytes, depending on the in vitro growth condition.69 The radiosensitivity, repair, and regeneration of OPCs in rat spinal cord have been characterized using an in vivo-in vitro clonogenic assay of these cells.24,70–72 This assay was used in a study of boron neutron capture therapy (BNCT) to rat spinal cord to determine the role of OPCs versus vascular damage in white matter injury. Results of this study will be discussed later. In the brain, cells that are susceptible to radiation-induced apoptosis are largely concentrated in the subependymal zone of the lateral ventricles and the subgranular layer of the dentate gyrus.43,73 These apoptotic cells are of particular interest, as neural progenitors and stem cells reside in the adult subependymal zone and dentate gyrus.74,75 Similar to the apoptotic response of oligodendrocytes, radiation-induced apoptosis of subventricular cells is p53 dependent and is virtually absent in transgenic mice knockout of the p53 gene.41 Neural stem cells with multipotential and self-renewing properties are present in adult spinal cord. They can be isolated and form neurospheres in vitro.19 Similar to the studies in OPCs,24,70–72 the radiation response of these neural progenitors has been described recently using in vitro neurosphere assay following in vivo irradiation of the rat spinal cord.25,76 This in vivo-in vitro assay can be used to describe the repair and repopulation of these neural stem cells/progenitors after irradiation. Recent studies suggest that cranial XRT was associated with inhibition of neurogenesis.45,46,77 In rat brain after a single XRT dose of 10 Gy, there was ablation of neurogenesis in the hippocampal dentate gyrus, an area where there is ongoing generation of new neurons, and it was observed that neural stem cells/progenitors instead differentiated into glial cells. Furthermore, the inhibition of neurogenesis was accompanied by disruption of the microvascular angiogenesis associated with neurogenesis, and an increase in the number and activation status of microglia was observed.78
Spinal Cord Radiobiology
 Structure of the Spinal Cord
Structure of the Spinal Cord
Anatomy
Cell Types
 General Radiobiological Concepts Pertinent to the Spinal Cord
General Radiobiological Concepts Pertinent to the Spinal Cord
Histopathogic Features of Spinal Cord Injury
Cellular and Molecular Mechanisms in Spinal Cord Myelopathy
Gene Induction and Oxidative Stress
Oligodendrocytes, Oligodendroglial Progenitors, and Demyelination
Role of Neural Stem Cells and Neural Progenitors
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


