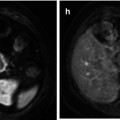
Fig. 27.1
Midsagittal post-contrast T1-weighted image shows a solid enhancing astrocytoma extending from the medulla to T4 that slightly expands the spinal cord
Astrocytomas tend to have an infiltrative appearance, while ependymomas are localized and well marginated and when small may be centrally located in the spinal cord.
Astrocytomas tend to be long tumors, while ependymomas are shorter; astrocytomas may be holocord or even multifocal.
Cysts tend to be more common in astrocytomas than in ependymomas. Cysts occurring at the rostral and caudal ends of the tumors are called “polar cysts” (Fig. 27.2). The presence of these polar cysts does not favor a specific type of tumor.
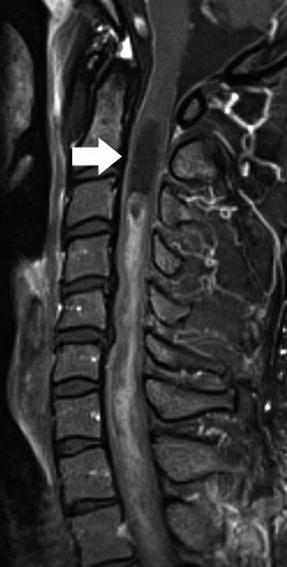

Fig. 27.2
Midsagittal post-contrast T1-weighted image shows a central and enhancing spinal cord astrocytoma extending from C3–T2 with a cephalad “polar” cyst (arrow)
Contrast enhancement is common in both and may not be used to different one from the other (Table 27.1). Rarely, astrocytomas may show no contrast enhancement.
Table 27.1
Most common spinal cord tumors
Type/histology | Diffuse | Localized |
|---|---|---|
Benign | Astrocytoma, ganglioglioma, oligodendroglioma | Ependymoma, hemangioblastoma, pilocytic astrocytoma |
Malignant | Anaplastic astrocytoma, glioblastoma, lymphoma | Metastases |
Older and newer blood products (including diffuse superficial siderosis) are more much common in ependymomas than in astrocytomas (Fig. 27.3).
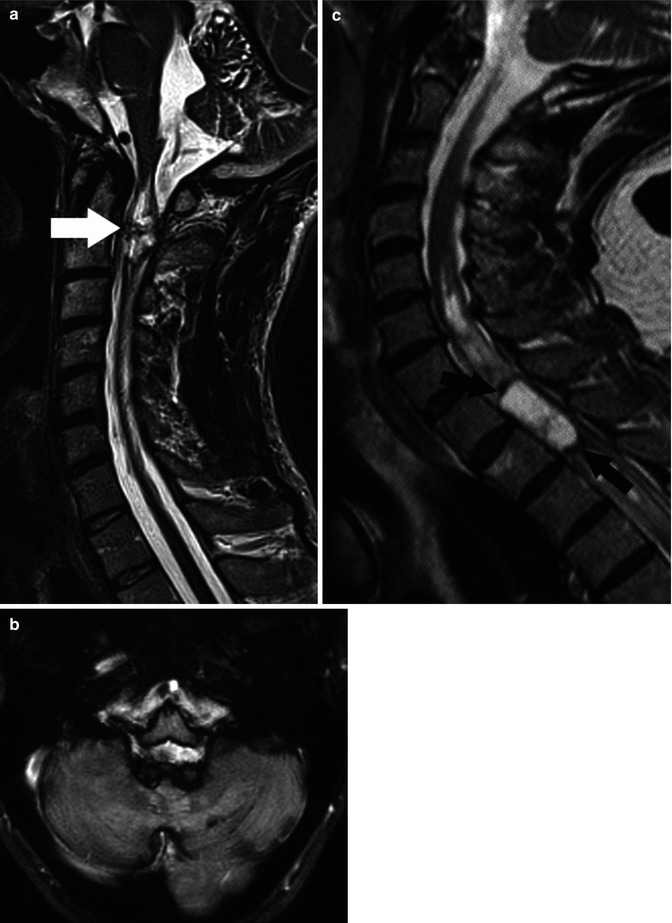

Fig. 27.3
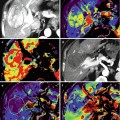
(a) Midsagittal T2-weighted image shows an ependymoma (arrow) containing dark regions which are related to chronic blood products and superficial siderosis on the cord, brainstem, and cerebellum. (b) Axial T2 image in the same patient clearly shows siderosis on all surfaces of the brain. (c) Midsagittal T2-weighted image in a different patient shows a mainly “cystic-” appearing ependymoma with marginal chronic hemorrhage seen as dark areas (arrows) (Courtesy J. Campo, Argentina)
Calcifications are uncommon in both tumors and generally not appreciated as tumors get mostly studied with MRI and not computed tomography.
Glioblastomas have no specific imaging features but are characterized by very rapid growth despite aggressive therapies [3] (Fig. 27.4).
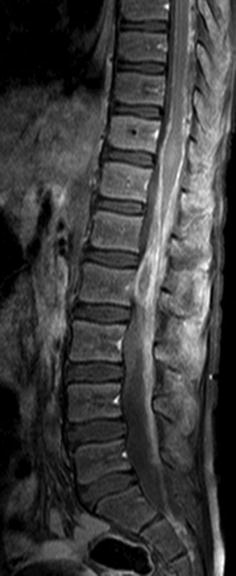

Fig. 27.4
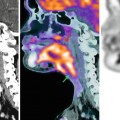
Midsagittal post-contrast T1-weighted image in a child with a proven diffuse cord glioblastoma shows enhancement of the cauda equina, conus medullaris, and pial surfaces of the cord. The findings are nonspecific (Courtesy A. Tellez, Chile)
Despite the fact that spinal cord ependymomas are more common in patients with NF-2, they still tend to be relatively rare even in that setting. Like all ependymomas, they arise from the ependymal cells – in this case those lining the central canal of the spinal cord (Fig. 27.5). They tend to have a slow and indolent clinical course and remain histologically of low grade, and their treatment is controversial and not well established. Ependymomas arising in NF-2 patients tend to be more common in males and involve predominantly the cervical cord and lower brainstem.
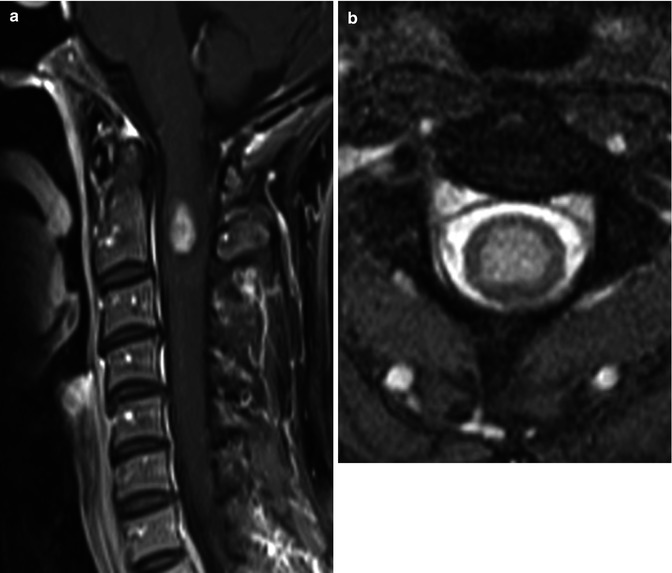

Fig. 27.5
(a) Midsagittal post-contrast T1 image shows a small centrally located enhancing ependymoma. (b) Axial T2 image in the same patient confirms the central location of the hyperintense mass which is typical of ependymoma
Although there are inherent technical difficulties in obtaining diffusion tensor imaging (DTI) tractography (AKA: fiber tracking) studies in the spinal cord, recently several publications have emphasized its usefulness [5–9]. Since the major predictor of prognosis and survival is tumor respectability, DTI may provide information not available by other diagnostic preoperative means. One of the most important information neuroimaging provides to the surgeon is if a tumor is localized and thus resectable or infiltrative and thus unresectable. Based on this information the neurosurgeon can plan for a multilevel laminectomy or a single one for the sole purpose of biopsy. In one study, 14 patients with spinal cord tumors underwent DTI using a 3.0 T unit [6]. Fiber course was evaluated and based on it tumors were classified as resectable or non-resectable and then compared with the intraoperative findings. If the lesions did not contain any fibers or if only a few fibers were visible within them, they were considered as resectable and at surgery all of them were ependymomas (Fig. 27.6). Lesions containing a multitude of fibers and considered non-resectable were proven to be astrocytomas, lymphomas, or multiple sclerosis (Figs. 27.7 and 27.8). These findings are similar to those of a smaller study in which DTI showed intra-tumoral fibers in five astrocytomas [9]. Interestingly, in this last study DTI was unable to differentiate peri-tumoral edema from true tumor infiltration (similar findings have been noted in the evaluation of brain tumors). In the study previously mentioned that included 14 patients with a variety of spinal cord lesions evaluated with DTI, the findings in one patient with a pilocytic astrocytoma also showed displacement of fibers only [6]. Thus, it seems that DTI holds promised in preoperatively separating resectable from non-resectable spinal cord tumors. It is also to be remembered that localizing tumor-associated cysts is also very important for the neurosurgeon. Any large cyst may need to be decompressed to ameliorate symptoms regardless if the tumor will be completely resected or only biopsied.
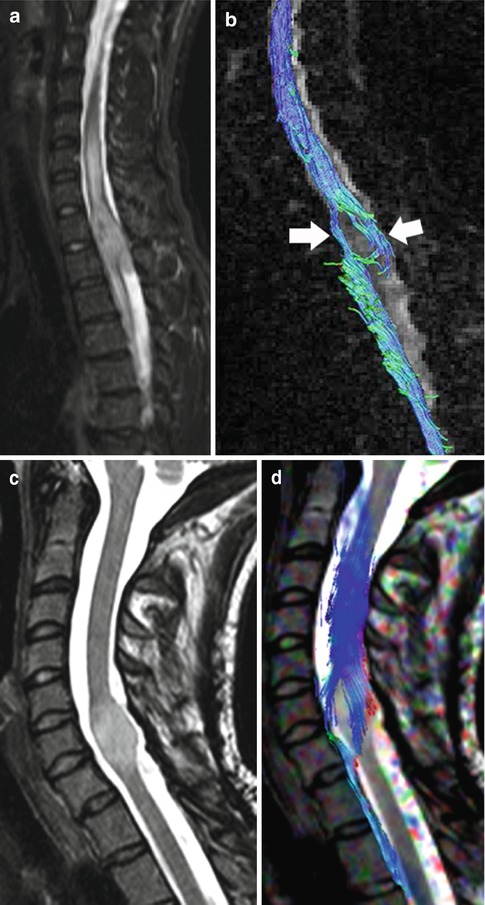
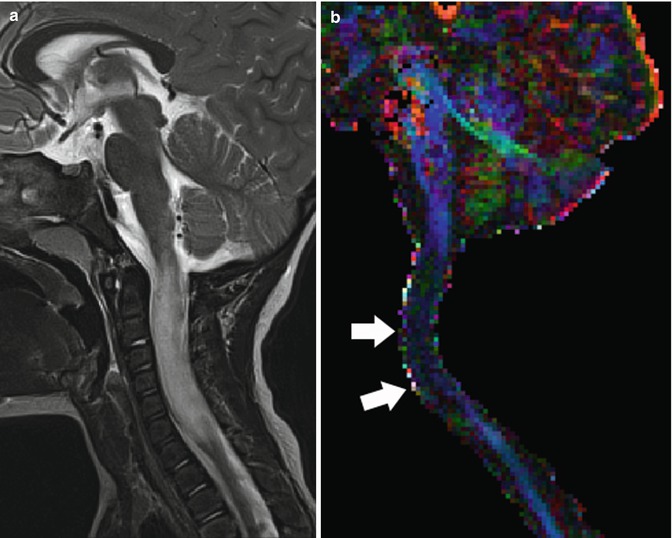
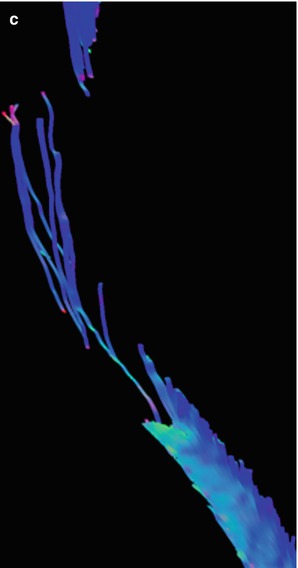
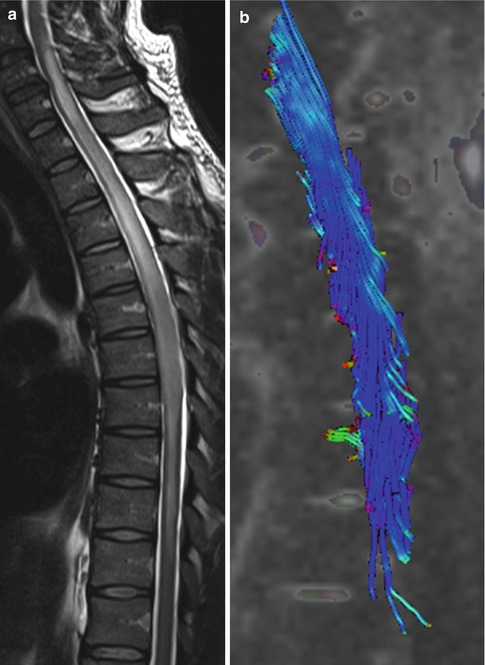

Fig. 27.6
(a) Sagittal T2-weighted image shows a mass in the cord at T1–T2 level with cephalad and caudal edema. (b) Fiber tracking DTI study shows no fibers traversing the tumor. The spinal cord fibers drape around the tumor (arrows) implying a localized, non-infiltrating mass, in this case an ependymoma. (c) In a different patient, sagittal T2 image demonstrates a focal, expansible, intramedullary, and bright tumor at C6–C7. (d) Corresponding fiber tracking DTI study showing that the cord’s white matter tracts wrap around this ependymoma and that the tumor contains no fibers (Courtesy of R. D. Murtagh, Florida)


Fig. 27.7
(a) Midsagittal T2-weighted image shows large intramedullary tumor with ill-defined borders from the medulla to C7. (b) Corresponding color-coded DTI directionality map shows loss of anisotropy (color) (arrows) in the region of the tumor due to fiber infiltration and lack of water motion directionality. (c) Corresponding fiber tracking DTI study shows that many fibers cross this infiltrative tumor (astrocytoma) making it unresectable

Fig. 27.8
(a) Midsagittal T2-weighted image shows ill-defined astrocytoma involving most of the thoracic spinal cord. (b) Fiber tracking DTI study in the mid-thoracic region shows that most white matter fibers cross the site of the tumor, and thus, it is unresectable
Because the traditional imaging features of most spinal cord tumors may overlap, it is important to be familiar with their differential diagnosis. Gangliogliomas are rare primary tumors that are much more common in children than in adults and account for only 1 % of all spinal cord masses [10]. These tumors are slow growing, a fact reflected by the long-standing symptoms (about 5 years in average) reported by the patients. Most spinal cord gangliogliomas are WHO grade II, but some are grade III (anaplastic) and their localized variant (grade I) corresponds to the gangliocytoma [1]. Other variants such as ganglioglioneurocytomas and glioneurofibroma are very rare and generally present before 3 years of age. There are no specific imaging features but most gangliogliomas are extensive (more than eight vertebral bodies in length) and tend to arise in the cervical region; thus, they are the most important alternative diagnosis to astrocytoma. Enhancement is perhaps less common and less intense than that seen in astrocytomas; calcifications may occur, but overall the diagnosis remains a histological one and they cannot be differentiated from astrocytomas by imaging alone.
Oligodendrogliomas arising in the spinal cord are very rare [1]. They tend to be found in younger patients and most of them are WHO grades II and III. Their imaging characteristics are nonspecific. These tumors are infiltrative in nature. Subependymomas are variants of ependymomas which more often arise in the cervical spinal cord [1]. Their imaging features are also nonspecific.
Other rare neoplasias to be included in the differential diagnosis include spinal cord lymphoma, sarcomas (which can be de novo or arise years after radiation therapy), and melanocytomas and melanomas (which may be of high signal intensity on pre-contrast T1-weighted images, a finding that suggests their nature) (Table 27.2) (Fig. 27.9).
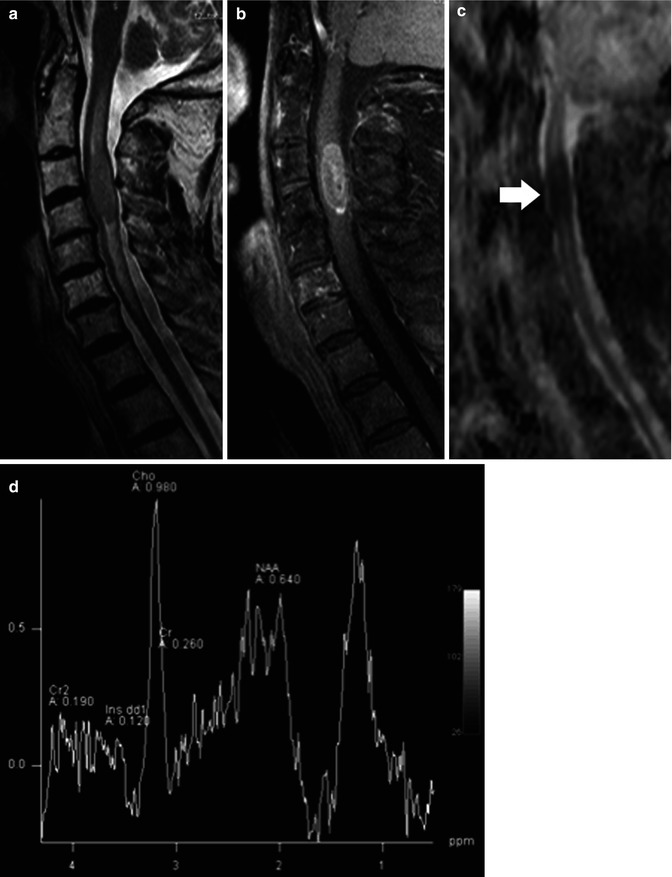
Table 27.2
Imaging features of most common spinal cord tumors including advanced modalities
Imaging feature/tumor type | Contrast enhancement | ADC values | DTI features | MRS |
|---|---|---|---|---|
Benign astrocytoma | Nearly always | High to normal | Fibers containedin tumor | High Cho, low NAA |
Malignant astrocytoma | Always | Low | Fibers containedin tumor | High Cho and low NAA, lipids, and lactate |
Ependymoma | Nearly always | High to normal | Fibers displacedby tumor | High Cho, low NAA |
Hemangioblastoma | Always | High to normal | Fibers displacedby tumor | High Cho, low NAA |
Lymphoma | Always | Low | Fibers containedin tumor | High Cho and low NAA, lipids, and lactate |
Metastases | Always | Low | Fibers displacedby tumor | High Cho and low NAA, lipids, and lactate |

Fig. 27.9
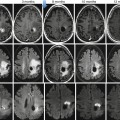
(a) Midsagittal T2-weighted image show a somewhat low signal intramedullary lymphoma at C3–C4 with surrounding edema. (b) Corresponding post-contrast T1 image demonstrates enhancement in the mass. (c) Corresponding ADC map shows that the lesion is of low signal (arrow) implying restricted diffusion to the high cellularity. (d) 1H-MR spectroscopy demonstrates elevated lipid peaks, a high choline/creatine ratio, and a low N-acetylaspartate/creatine ratio (reprinted with permission from Hygino da Cruz LCH [11])
27.3 Hemangioblastoma and Other Localized Tumors
Localized tumors are focal lesions in which MRI shows an enhancing mass or nodule which is clearly separable from the adjacent spinal cord. The category of localized tumors is broad and includes distinctively different lesions such as primary and secondary (metastases) tumors, and they are addressed here as a group because the first and upmost goal of treatment is complete resection. Ependymoma and subependymoma are localized tumors, but they have been previously discussed and for the sake of brevity will not be addressed again in this section. For purposes of this discussion, we will classify localized spinal cord masses as:
Primary:
(a)
True tumors: ependymoma, hemangioblastoma, pilocytic astrocytoma, and intramedullary schwannoma
(b)
Non-tumoral masses: lipoma, dermoid, epidermoid, and cavernous malformations
Secondary: Metastases
Hemangioblastomas are of uncertain origin and represent the third most common primary intramedullary spinal cord tumor after astrocytoma and ependymoma accounting for nearly 7 % of cord masses [1]. Unlike other primary cord tumors, there is no gender predilection and their age of discovery is about 40 years. Nearly 70 % of them are solitary and about 30 % occur in combination with the von Hippel-Lindau (VHL) syndrome. About 70 % of VHL patients develop central nervous system hemangioblastomas during their lifetime. The most common tumor location is the cervical cord followed by the thoracic cord. Twenty-five percent of them occur on the surface of the cord and the rest inside the cord. A finding that is critical is that all hemangioblastomas touch a pial or ependymal surface (Fig. 27.10). They are highly vascular, a fact that leads to visualization of intra-tumoral blood vessels in those measuring over 1 cm, and all demonstrate intense contrast enhancement. Associated edema and cysts are quite common and it is said that it is the cyst rather than the tumor that produces the clinical symptoms; thus, imaging mapping of the cyst extent is critical. They may bleed and occasionally result in superficial siderosis. The differential diagnosis includes pilocytic astrocytoma which may look identical but lacks the flow voids (arteries and veins) present inside hemangioblastomas.
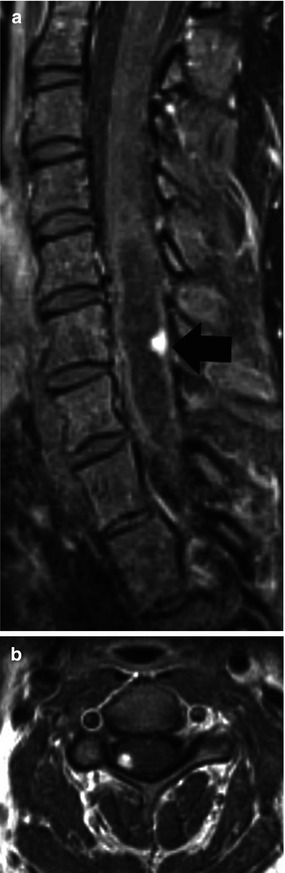

Fig. 27.10
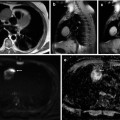
(a) Midsagittal post-contrast T1-weighted image shows a small enhancing hemangioblastoma (arrow) near the surface of the cord with accompanying cyst. (b) Axial post-contrast T1 image clearly shows the subpial location of the tumor
Since the spinal cord contains no Schwann cells, the origin of intramedullary schwannoma has been hotly debated [12–14]. It is thought that these rare tumors arise from the perivascular nervous plexus of pial blood vessels. These tumors are extremely rare (0.3 % of all spinal cord tumors) and have no specific imaging findings generally presenting as small enhancing nodule(s) inside the cord in its dorsolateral aspects. Curiously schwannomas may be accompanied by edema and the presence of an adjacent enlarged and enhancing nerve root strongly suggests the diagnosis. Neurofibromas may also arise in the spinal cord but are even less common than schwannomas and have no specific imaging findings.
Of the non-tumoral masses, lipoma, dermoid, and epidermoid are generally extramedullary lesions, but occasionally they may arise inside the cord [15–17]. All three lesions may be associated with underlying spinal dysraphisms and may show high signal on DWI and restricted diffusion similar to their intracranial counterparts [1, 18




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree