ASTRO guidelines for general spine SBRT (2011)
■ Life expectancy ≥3 months
■ Limited disease burden
■ Previously radiated location(s)
■ Postoperative radiation
■ Favor enrollment on a clinical trial
Spinal cord compression
■ Limited compression (1–2 segments)
■ Sub-acute presentation (outcome unlikely to be impacted by protracted SBRT planning)
■ Re-irradiation
Primary spinal cord neoplasms
■ Postoperative adjuvant setting
■ Salvage
Workup
H&P with emphasis on neurologic components.
Review of systems , including:
Focal weakness.
Focal sensory changes .
Bowel or bladder incontinence , and perianal numbness which could indicate cauda equina involvement.
Back pain .
Laboratories not typically required, except in cases where adjacent viscera may be invaded or if there is concern for hematologic malignancy (then CBC , CMP , LFTs, etc.).
Imaging.
MRI spine with gadolinium remains the gold standard for assessment of spinal cord neoplasms, and is also critical for SBRT targeting.
CT myelogram (standard or metrizamide -enhanced) is often useful in patients with metallic vertebral implants or a permanent pacemaker . At some institutions, CT myelograms are standard practice for spine SBRT planning.
MRI neurogram may be used to assess for nerve root involvement but has limited utility in SBRT planning.
Radiosurgical Technique
Simulation and Treatment Planning
Invasive stereotactic frames that attach to spinous process es (Hamilton and Lulu 1995; Hamilton et al. 1995) have fallen out of favor with the advent of noninvasive immobilization device s that allow for targeting accuracy within 1–2 mm and 1–2° (Ryu et al. 2003; Yenice et al. 2003).
Fluoroscopic placement of percutaneous gold fiducial markers into vertebral pedicles can be used to enhance intrafraction tumor targeting and tracking, but spinal tracking is most often sufficient.
Insertion of a percutaneous balloon into pre-sacral space may be considered to displace the rectum if needed for definitive treatment of complex sacral lesions.
CT simulation with slice thickness ≤3 mm (1–1.5 mm recommended).
MRI and/or CT myelogram should be used in patients with vertebral hardware.
Co-registration with MRI or PET /CT images when available.
Target volumes:
GTV : Residual disease on CT/MRI .
CTV : GTV plus postoperative bed at high risk for recurrence.
PTV : CTV + 1.5–2 mm margin excluding critical neural structures.
Dose Prescription
No randomized studies are available to provide firm recommendations for dose selection, and a clear dose-response relationship for pain control has not been established. However, there is a trend for symptomatic improvement (Ryu et al. 2003, 2007; Gerszten et al. 2006) and improved control of radioresistant histologic subtypes with increased dose (Gerszten et al. 2005a, b; Yamada et al. 2008).
Limited disease in patients without prior radiation: 16–24 Gy in 1 fraction.
Multi-segment disease without prior radiation: 20–27 Gy in 2–3 fractions.
Multi-segment disease in previously irradiated field: 20–25 Gy in 5 fractions.
Chordoma : 40 Gy in 5 fractions (UCSF experience).
Dose Delivery
For multifraction regimens, doses are delivered every other day or twice weekly.
Initial verification by kV X-ray or CBCT , aligned to spine or surrogate fiducial markers of position.
Interval verification during treatment delivery with repeat kV X-ray films or CBCT for longer treatments or patients unable to remain immobile (Figs. 5.1, 5.2, and 5.3).
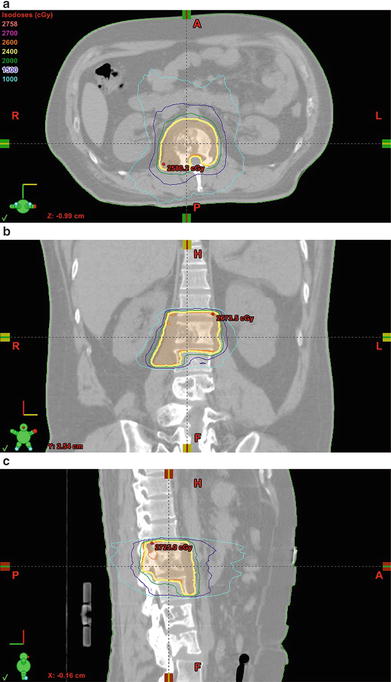
Fig. 5.1.
SBRT for vertebral body metastasis . (a–c) Thirty-nine year-old male with stage IVC nasopharyngeal carcinoma and a painful L1 vertebral body metastasis extending to the bilateral epidural space and right psoas muscle . The metastasis was treated with rapid arc stereotactic radiosurgery to a total dose of 2400 cGy in a single fraction with 6 MV photons prescribed to the 87 % isodose line
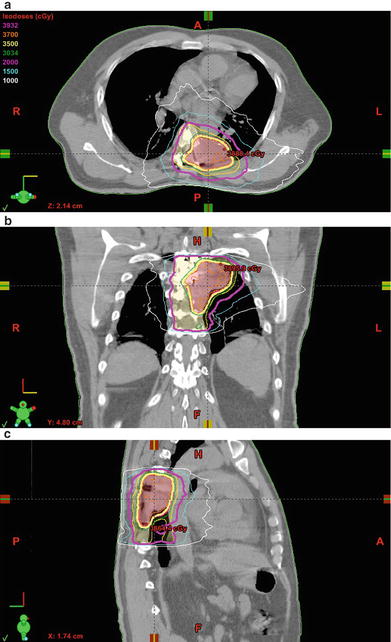
Fig. 5.2.
Postoperative SBRT for primary spine tumor. (a–c) Forty-nine year-old male with a remote history of medullary thyroid cancer who subsequently developed a painful left posterior 7th rib lesion that was treated with a course of palliative radiotherapy to 3300 cGy in 11 fractions at an outside institution. The lesion continued to grow over the following 2 years, and a biopsy demonstrated chondrosarcoma . Following gross total resection and two subsequent recurrences, the GTV was treated with rapid arc stereotactic radiosurgery to a total dose of 3500 cGy in 5 fractions, with 2000 cGy to the postoperative bed, using 6 MV photons prescribed to the 88 % isodose line
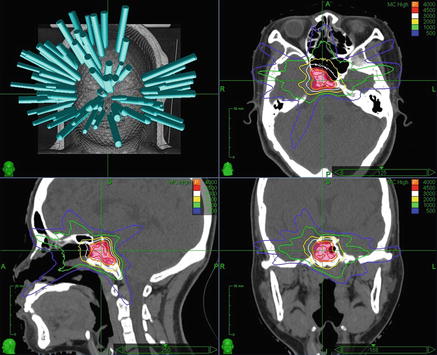
Fig. 5.3.
Clival chordoma SBRT . Thirty year-old female with a clival chordoma status post gross total endoscopic endonasal transsphenoidal resection , followed by repeat gross total resection for a recurrence 1 year later. The tumor was treated with adjuvant robotic radiosurgery to a total dose of 4000 cGy in five daily sequential fractions with 6 MV photons prescribed to the 83 % isodose line . Beam angles are shown at the top left, and proceeding clockwise are axial, coronal, and sagittal CT images with isodose lines and the PTV in red color wash
Toxicities and Management
Acute toxicities (≤6 weeks):
Low risk of acute, self-limited esophagitis , nausea /vomiting , and loose stool with treatment of cervicothoracic, lumbar, and sacral spinal lesions, respectively; manage with antiemetic and antidiarrheal agents.
Cutaneous toxicities are rare, mild, and generally limited to treatment of lesions extending into the posterior paraspinous space.
Late toxicities (>6 weeks):
Vertebral body compression fracture is a fairly low-risk adverse event after conventional radiotherapy (~5 %), but estimates range from 11 to 39 % after spine SBRT (vide infra).
Serious late effects to the esophagus and bronchi , such as necrosis and ulceration , are rare but may require surgical intervention.
Late toxicity to the brachial plexus , lumbar plexus and spinal cord , including both self-limited myelopathy and chronic progressive myelopathy, are similarly uncommon and may be mitigated with hyperbaric oxygen treatment.
Lhermitte’s syndrome, an electric sensation running down the back into the limbs, often precedes frank neurologic deficits of radiation myelopathy .
Recommended Follow-Up
H&P and MRI spine every 2–3 months or as clinically indicated for the first 2-years, followed by imaging every 6 months for the next 3 years, and yearly imaging thereafter.
Evidence
Dose and Technique
Yamada et al. (2005): Noninvasive immobilization for paraspinal stereotactic or image-guided radiotherapy with setup accuracy within 2 mm. Thirty-five patients (14 primary tumors and 21 metastases) with gross disease involving the spinal canal who were either previously irradiated or treated with doses beyond conventional spinal cord tolerance. PTV = gross disease with a 1 cm margin, excluding the spinal cord. For primary treatments, median PTV dose 7000 cGy in 33 fractions with V100 of 90 %; median cord Dmax 68 %. In re-irradiation cases, median PTV dose 20 Gy in 5 fractions with V100 of 88 %; median cord Dmax 34 %. Median follow-up 11 months; no radiation myelopathy . Palliation from pain , weakness, or paresis in 90 % of patients with >3 months of follow-up. LC 75 and 81 % for secondary and primary malignancies, respectively.
Chang et al. (2007): Prospective phase I/II study of SBRT for spinal metastases in 63 patients with 74 tumors treated at MDACC (30 Gy in 5 fractions or 27 Gy in 3 fractions; spinal cord Dmax ≤10 Gy). In previously radiated patients (n = 35, 56 %), prior dose ≤45 Gy. Median follow-up 21.3 months; no neuropathy or myelopathy . Actuarial 1-year PFS 84 %. Primary mechanisms of failure limited to recurrence in adjacent bones (i.e., pedicles and posterior vertebral elements), and epidural space. Narcotic usage declined from 60 to 36 % at 6 months.
Ryu et al. (2008): Forty-nine patients with 61 separate spinal metastases treated with single-session SBRT from 10 to 16 Gy. Spinal cord limited to ≤10 Gy for ≤10 % of the cord volume 6 mm superior and inferior to the treated segment. Median time to pain relief 14 days (earliest within 24 h). Complete pain relief in 46 % and partial relief in 19 %. Overall pain control rate for 1 year was 84 %; median duration of relief 13.3 months. Trend toward increasing pain relief with ≥14 Gy. No clinically detectable late toxicity .
Yamada et al. (2008): One-hundred three consecutive spinal metastases in 93 patients treated with 18–24 Gy in 1 fraction (median 24 Gy) prescribed to the 100 % isodose line ; spinal cord Dmax ≤14 Gy. Patients with high-grade cord compression, mechanical instability, and prior history of RT excluded. Median follow-up and OS both 15 months; actuarial LC 90 % with median time to LF 9 months. Radiation dose, but not histologic subtype, was a significant predictor of LC. Acute toxicity limited to grade ≤2 events; no late toxicity . All patients without local failure reported durable palliation of symptoms.
Amdur et al. (2009): Prospective phase II study of SBRT for spinal cord metastases involving 25 sites in 21 patients treated with 15 Gy in 1fraction. Primary endpoint was toxicity; spinal cord Dmax ≤12 Gy in patients with no prior radiotherapy (n = 9), and ≤5 Gy for salvage cases (n = 12). With median follow-up 11 months, 95 % LC and 43 % pain improvement, but 1-year OS 25 % and PFS 5 %. Acute toxicity limited to grade ≤2 dysphagia or nausea ; no late toxicity .
Spinal Cord Compression and Retreatment
Milker-Zabel et al. (2003): Eighteen patients with 19 previously irradiated spinal cord metastases (median dose 38 Gy) re-treated due to progressive pain (n = 16) or neurologic symptoms (n = 12). Median time to re-treatment 17.7 months. Five patients treated with fractionated conformal radiotherapy (FCRT ), 14 treated with IMRT ; all immobilized for extracranial stereotaxy. Median re-treatment dose 39.6 Gy in 2 Gy fractions. After median of 12 months of follow-up, OS 65 %, LC 95 %, pain relief 81 %, and neurologic improvement 42 %. Tumor size unchanged in 84 % of cases. No clinical late toxicity .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree