This content presents infectious and vascular spinal emergencies, including epidural abscess, nontraumatic epidural hematoma, vascular malformations, and spinal cord infarction. The spine is subjected to multiple potential insults, such as trauma, infection, ischemia, hemorrhage, tumor, inflammation, and degeneration. All of these processes can lead to the sudden onset of neurologic symptoms, such as motor weaknesses, bowel and bladder incontinence, and sensory changes. Therefore, prompt recognition of these entities is important to reverse or minimize potential neurologic injury. The authors discuss several infectious and vascular spinal emergencies, including epidural abscess, nontraumatic epidural hematoma, vascular malformations, and spinal cord infarction.
Epidural abscess
Spinal epidural abscess can develop primarily or after spinal surgery. Primary spinal epidural abscess is a rare entity, with an estimated prevalence of 0.2 to 2.0 cases per 10,000 hospital admissions. However, its incidence has increased over the last 20 years as a result of better diagnostic methods, an aging population, an increase in the number of patients with medical comorbidities, and an increase in intravenous drug use. Most cases occur in middle-aged to elderly adults, with a predilection for men. Risk factors include diabetes, intravenous drug use, previous invasive procedures to the spine, superficial infections of the back, immunocompromised state, and infections at distant sites. Staphylococcus aureus is the most common pathogen and is found in approximately 70% of cases. Gram-negative rods were previously reported to be associated with a significant number of spinal epidural abscesses and vertebral osteomyelitis; however, its prevalence has been decreasing over the years. In rare cases, other pathogens, such as tuberculosis, fungal, and parasitic agents, can also be associated with a spinal epidural abscess.
The classic clinical triad of fever, back pain, and neurologic deficits is found in only a small fraction of patients. Almost all patients have back pain, but fever occurs in only about two-thirds of patients, and neurologic deficits occur in 25% to 60% of patients. Therefore, many patients do not seek medical treatment with the onset of symptoms and a delay in diagnosis is not uncommon.
In terms of location, epidural abscess can occur in all 3 regions of the spine (cervical, thoracic, and lumbosacral). Of the 3 regions of the spine, the thoracic and the lumbosacral regions are affected more commonly than the cervical spine. There are also cases of a spinal epidural abscess involving the entire spine.
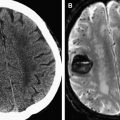
The spinal epidural abscess can cause neurologic deficits by either direct compression or by vascular compromise through thrombophlebitis or thrombosis. Thus, sudden neurologic deterioration can occur and prompt diagnosis is important. MR imaging is the initial study of choice for identifying a spinal epidural process of any etiology. In the case of an abscess, the diagnosis is often straightforward: isointense or hypointense on T1-weighted images and hyperintense on T2-weighted images. The collection usually partially enhances with gadolinium-based intravenous contrast media (Gad). The fluid portion of the abscess is typically hyperintense on T2-weighted images. The enhancement pattern sometimes helps to define the consistency of the collection. Specifically, liquid purulent material is associated with a central area of low T1 signal intensity; whereas, a rim of tissue that enhances after Gad represents granulation tissue. Recently, diffusion-weighted imaging (DWI) has been used in the spine; on DWI, the abscess is markedly hyperintense to the surrounding tissue and hypointense on the apparent diffusion coefficient map ( Fig. 1 ). More than 80% of patients with spinal epidural abscess have concomitant osteomyelitis. Therefore, in patients with a suspected spinal epidural abscess, it is important to look for additional findings of osteomyelitis or diskitis.
Patients who are unable to undergo MR imaging can be studied with CT myelography (CTM), which has been shown to have similar sensitivity to MR imaging for detecting an epidural abscess. MR imaging has the advantage of being more specific in its ability to differentiate the epidural mass from other pathologies. In addition, it is superior for evaluating the adjacent paraspinal soft tissues. Moreover, CTM requires intrathecal injection of contrast material and therefore has the theoretical risk of introducing the infectious agents into the intrathecal space in cases of lumbosacral abscess. Nevertheless, CTM may be an alternative diagnostic method for patients who are unable to undergo an MR imaging examination and for patients in whom MR imaging may be difficult to interpret because of previous spinal instrumentation. Although MR imaging, and to a lesser extent CTM, is a key diagnostic study to establish the diagnosis of a spinal epidural abscess, plain radiographs or CT of the spine are often the first diagnostic tests that are obtained, especially in patients who are afebrile without neurologic deficits. Surgical decompression with antibiotic treatment have historically been the mainstay of treatment for spinal epidural abscess. Recently, nonsurgical treatment has been used to treat patients with no neurologic deficits, significant comorbidities, or complete spinal cord injury at the affected level below the level of epidural abscess.
Epidural hematoma
Spinal epidural hematoma (EDH) can compress the spinal cord or nerve roots and result in neurologic deficits. Spinal EDHs occur most commonly after spinal surgery. After discectomy or decompression, EDH can be identified in 33% to 100% of the patients when evaluated by postoperative CT or MR imaging. However, despite the radiographic appearance of compression by many of these lesions, neurologic symptoms associated with postoperative EDH are extremely rare. It is estimated that the incidence of symptomatic EDH after spinal surgery is only 0.1% to 0.2%. Risk factors associated with EDH formation include advanced patient age, multilevel surgery, and coagulopathy (increased international normalized ratio).
In contrast, spontaneous EDHs of the spine are rare. The incidence of spontaneous spinal epidural hematomas is estimated to be 0.1/100,000 population per year. They usually occur in older patients (aged 50–80 years) with a slight predominance of male patients. Limited evidence suggests that the spontaneous spinal EDH is caused by bleeding from the valveless venous plexus, although some investigators postulate that arterial rupture may be the cause. In most cases, there is no clear source of hemorrhage or a causative event. There have been several case reports of spinal epidural hematomas occurring after chiropractic manipulation, epidural anesthesia, and steroid injection. The incidence of spinal EDH after chiropractic manipulation is extremely low, with fewer than 10 cases reported in the literature. The risk of spinal EDH after epidural anesthesia is also low, estimated to be about 1:220,000. It is thought that coagulopathy increases the risk of spinal EDH in these latter cases.
The classic clinical presentation of a spinal EDH consists of the sudden onset of back or neck pain. Neurologic symptoms related to the compression of nerve roots or spinal cord may also occur. Many pathologic conditions, including intervertebral disc herniation, infection, pathologic fractures associated with tumor, transverse myelitis, and other vascular malformations, may mimic the clinical presentation of a spinal EDH. Therefore, prompt evaluation with MR imaging is important to establish the diagnosis.
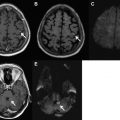
The typical imaging appearance of an acute spinal EDH is that of a homogenously isointense to hyperintense mass on T1-weighted images, and hyperintensity on T2-weighted images. The hematoma can have homogenous or heterogeneous hyperintensity on T2-weighted images. Atypical appearance of the hematoma usually involves a shift from isointense to hyperintense on T1-weighted images. Importantly, the acute spinal EDH should not enhance with gadolinium-based intravenous contrast media. In one series of 19 subjects with a diagnosis of spinal EDH, 2 cases showed Gad enhancement on MR imaging and the intraoperative findings revealed that neither case was an EDH. Most hematomas occur in the cervical or the thoracic region ( Fig. 2 ).
In the majority of cases, emergent surgical evacuation of the hematoma is the treatment of choice. Patients with small hematomas and without neurologic deficit may be managed conservatively. For patients with neurologic deficits, it is important to remove the hematoma. Further, because neurologic improvement has been observed even in patients presenting with complete injury, prompt recognition, diagnosis, and treatment are essential for the optimal management of the spinal EDH.
Epidural hematoma
Spinal epidural hematoma (EDH) can compress the spinal cord or nerve roots and result in neurologic deficits. Spinal EDHs occur most commonly after spinal surgery. After discectomy or decompression, EDH can be identified in 33% to 100% of the patients when evaluated by postoperative CT or MR imaging. However, despite the radiographic appearance of compression by many of these lesions, neurologic symptoms associated with postoperative EDH are extremely rare. It is estimated that the incidence of symptomatic EDH after spinal surgery is only 0.1% to 0.2%. Risk factors associated with EDH formation include advanced patient age, multilevel surgery, and coagulopathy (increased international normalized ratio).
In contrast, spontaneous EDHs of the spine are rare. The incidence of spontaneous spinal epidural hematomas is estimated to be 0.1/100,000 population per year. They usually occur in older patients (aged 50–80 years) with a slight predominance of male patients. Limited evidence suggests that the spontaneous spinal EDH is caused by bleeding from the valveless venous plexus, although some investigators postulate that arterial rupture may be the cause. In most cases, there is no clear source of hemorrhage or a causative event. There have been several case reports of spinal epidural hematomas occurring after chiropractic manipulation, epidural anesthesia, and steroid injection. The incidence of spinal EDH after chiropractic manipulation is extremely low, with fewer than 10 cases reported in the literature. The risk of spinal EDH after epidural anesthesia is also low, estimated to be about 1:220,000. It is thought that coagulopathy increases the risk of spinal EDH in these latter cases.
The classic clinical presentation of a spinal EDH consists of the sudden onset of back or neck pain. Neurologic symptoms related to the compression of nerve roots or spinal cord may also occur. Many pathologic conditions, including intervertebral disc herniation, infection, pathologic fractures associated with tumor, transverse myelitis, and other vascular malformations, may mimic the clinical presentation of a spinal EDH. Therefore, prompt evaluation with MR imaging is important to establish the diagnosis.
The typical imaging appearance of an acute spinal EDH is that of a homogenously isointense to hyperintense mass on T1-weighted images, and hyperintensity on T2-weighted images. The hematoma can have homogenous or heterogeneous hyperintensity on T2-weighted images. Atypical appearance of the hematoma usually involves a shift from isointense to hyperintense on T1-weighted images. Importantly, the acute spinal EDH should not enhance with gadolinium-based intravenous contrast media. In one series of 19 subjects with a diagnosis of spinal EDH, 2 cases showed Gad enhancement on MR imaging and the intraoperative findings revealed that neither case was an EDH. Most hematomas occur in the cervical or the thoracic region ( Fig. 2 ).
In the majority of cases, emergent surgical evacuation of the hematoma is the treatment of choice. Patients with small hematomas and without neurologic deficit may be managed conservatively. For patients with neurologic deficits, it is important to remove the hematoma. Further, because neurologic improvement has been observed even in patients presenting with complete injury, prompt recognition, diagnosis, and treatment are essential for the optimal management of the spinal EDH.
Arteriovenous shunts
Arteriovenous shunts and vascular malformations in the spinal cord region are classified based on their nidus location, vascular supply, and drainage pattern. In one of the most commonly used classifications, developed by Oldfield and Doppman, the lesions are divided into 4 types:
Type I lesions are the typical dural arteriovenous fistula (DAVF), and usually arise near nerve roots.
Type II lesions are glomus arteriovenous malformations (AVM), with a mass of dysmorphic arteries and veins without an intervening capillary bed. These lesions can be partial or entirely intramedullary.
Type III lesions are juvenile AVMs that are composed of a network of arteries and veins, without an obvious nidus. These lesions can be extensive and involve the spinal cord, spine, and paravertebral tissue.
Type IV lesions are perimedullary DAVF.
Combined, these 4 types of arteriovenous shunts or vascular malformations account for 3% to 16% of all space-occupying lesions of the spinal cord, with the Type 1 DAVF being the most common. Each of these types are subsequently discussed.
Spinal vascular malformations/arteriovenous shunts can cause neurologic symptoms by 3 mechanisms:
- 1.
These lesions can cause acute neurologic symptoms by hemorrhage, and the risk of hemorrhage varies for the 4 types of lesions.
- 2.
Spinal vascular malformations and arteriovenous shunts can result in venous hypertension. Venous hypertension, in turn, can lead to reduced perfusion, and thus ischemia.
- 3.
They can cause symptoms by direct mass effect, resulting in a progressive myelopathy or radiculopathy.
In one study of 78 subjects with myelopathy of unknown cause, 22 subjects had an AVM on angiography. Importantly, 5 subjects in this series had previous MR imaging that was interpreted as normal.
Type I: Dural Arterial Venous Fistula
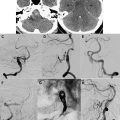
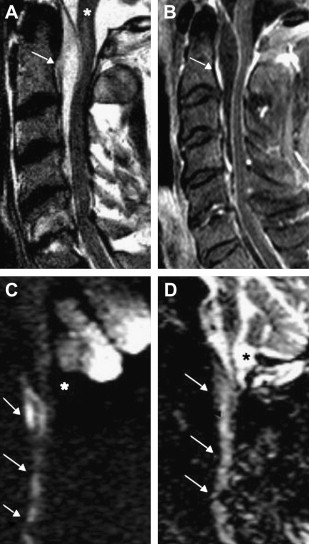
The spinal dural arterial venous fistula is an acquired arteriovenous shunt in the dura. It is most commonly located near the exiting nerve root. In some cases, the fistulous vessel may penetrate the dura at a more distant site and have a significant extradural component. Spinal DAVFs usually present in adults after the fourth or the fifth decade, have a significant male predominance, and most commonly affect the thoracic spine. Patients often present with vague complaints of back pain or radicular pain, followed by progressive lower-extremity paresis, and bowel and bladder sphincter dysfunction. Hemorrhage is extremely rare. Histologically, spinal DAVFs are supplied by a normal dural branch artery and drain into a single, dilated vein. This drainage results in retrograde flow into the venous plexus surrounding the spinal cord. Physiologic studies have shown that venous pressure is approximately 75% of systemic arterial pressure. The resultant venous hypertension and venous congestion cause a reduction in the arterial-venous pressure gradient, and thus a reduction in tissue perfusion pressure. A reduction in tissue perfusion pressure then leads to hypoxia, and further vasodilation may occur as a result of autoregulation. Eventually, compensatory mechanisms from autoregulation are exhausted, the spinal cord tissue becomes ischemic. Cord edema and progressive loss of function then develops.
Although most patients present with progressive myelopathy , acute worsening of motor or sphincter function can also occur. It is important to evaluate patients with an unclear cause of myelopathy, because 50% of untreated patients with a spinal DAVF can become disabled in 3 years. MR imaging is the initial screening test of choice, and it is important to obtain a whole-spine MR imaging survey because there can be a discrepancy between the location of the spinal DAVF and the spinal level as suggested by clinical signs and symptoms. The most common finding on MR imaging in patients with DAVF is abnormal T2 signal hyperintensity within the cord. There can also be enlargement of the cord, consistent with swelling from cord edema. The T2 signal changes can be extensive, extending over 6 to 7 vertebral levels in some cases. Occasionally, prominent, lacelike tortuous and ectatic flow voids may be seen. There can also be nonspecific enhancement with Gad contrast ( Fig. 3 ).