279CHAPTER 18
Spine SBRT
Advances in radiation therapy treatment planning and delivery have paved the way for increasing clinical implementation of extracranial stereotactic body radiation therapy (SBRT), with spine SBRT representing the second most common indication for SBRT across both academic and private practices in the United States (1). Although the oncological community awaits the completion and publication of a number of important international comparative effectiveness randomized clinical trials, such as RTOG 0631 and UK DOSIS (2, 3), a growing body of literature continues to evolve supporting the role of spine SBRT in a number of clinical indications including the following:
1. De novo SBRT as an alternative to primary conventional external beam radiation therapy for previously untreated spine metastases
2. SBRT as salvage treatment for previously irradiated spine metastases
3. Postoperative SBRT following surgical decompression with or without surgical stabilization
4. Decompressive SBRT as an alternative to surgical decompression for spinal epidural metastases
5. SBRT as a nonoperative alternative for the definitive treatment of primary intradural extramedullary spine tumors
De Novo SBRT
The improvements in systemic therapy, including the emergence of targeted therapies and immunotherapy, have created an emerging challenge in the treatment of stage IV cancer. Improved systemic therapy has significantly increased overall survival and improved clinical performance in patients with stage IV solid malignancies, thereby creating an increasingly important role for local therapy to high-risk lesions that occur in otherwise stable patients. This challenge places an increased emphasis on effective long-term treatments of spine metastases and spinal cord compression to mitigate the associated pain and risks of compromised neurological function. Modern radiation oncology and neurosurgical literature have highlighted the extended survival expectations for certain patients with spinal metastasis. Subgroups with a long interval from primary diagnosis, younger age at presentation, high performance status, and primary histologies with favorable systemic treatment options generally demonstrate a median survival in the range of 20 to 30 months (4, 5). These prognostic data should mitigate any high expectations of conventional techniques for the treatment of bone metastases or spine cord compression. The UK/New Zealand Bone Metastases randomized controlled trial, one of the many comparing low-dose single-fraction 8 Gy with conventional fractionated external beam radiation therapy of 20 Gy in five fractions or 30 Gy in 28010 fractions, showed a 40% to 50% probability of pain increase at 1 year irrespective of fractionation for uncomplicated bone metastases (6). Similarly, the landmark randomized clinical trial by Patchell et al., which established direct surgical decompression plus postoperative radiation therapy as standard of care over radiation therapy alone for malignant spinal cord compression, had a median time of ambulation for the winning arm of only 122 days with an estimated 1-year ambulation rate of 30% to 40% (7). When juxtaposed to the increasing survival of patients with stage IV cancer, the limited efficacy of conventional radiation therapy suggests that it is likely not an acceptable treatment modality for the 21st-century management of spine metastases. Further limiting the expectations with conventional irradiation is the impact of radiosensitivity, in which the median duration of response for radiosensitive histologies (e.g., breast, prostate, and hematological malignancies) is 11 months versus 3 months for radioresistant histologies (sarcoma, melanoma, renal cell carcinoma), and the estimated 2-year local control is 86% versus 30% (8, 9). With subgroups of patients with metastatic cancer surviving longer than the pain relief and tumor control provided by conventional techniques, SBRT has opened the door to a treatment option with potentially more effective and longer lasting treatment for spine metastases. A number of clinical series have been published highlighting the benefits of primary SBRT for de novo treatment of spine metastases in favorable subgroups, including rapid and durable pain relief with 1-year pain control rates of 85% to 95%, lasting effective tumor control in the 80% to 90% range, and cost-effective treatment over conventional irradiation (10–14).
Salvage SBRT for Previously Irradiated Spine Metastases
With increasing survival and limited long-term control and pain relief of conventional irradiation, a number of patients will present with recurrent or progressive spine metastases after prior irradiation. Historically, the application of reirradiation was limited because of concerns of spinal cord myelopathy, and lower reirradiation doses were often required to mitigate the risks posed by reirradiation with conventional radiation therapy (15). SBRT with sharp dose gradients permits delivery of high-dose radiation to the target lesion, while sparing the adjacent spinal cord in spine metastases that were irradiated earlier (16). Detailed multi-institutional analyses have confirmed the safety of reirradiation with SBRT following prior external beam radiation therapy, in which five cases of radiation myelopathy following SBRT were confirmed and compared with patients treated with SBRT not experiencing radiation myelopathy, providing practitioners objective dosimetry to guide safe application of spine SBRT (17). This recommendation for safe treatment with SBRT has similarly been made for spinal cord tolerance in the reirradiation setting. The guidelines assume a minimum of a 5-month reirradiation interval, SBRT comprising less than 50% of the total cord dose, and a SBRT cord maximum biologically effective dose 2 Gy (BED2 Gy) of 20 to 25 Gy and cumulative cord BED2 Gy of 70 Gy (measured at the thecal sac adding in a planning risk volume margin of 1.5 mm for safety margin, cord uncertainty in fusion, contouring, and motion). This guideline is generally considered safe and in line with prior reirradiation dose models (17).
Postoperative SBRT
For nonmoribund patients with intermediate- to high-grade neurologic symptoms, defined as paraparesis 4/5 or less, loss of ambulation, cauda equina syndrome, or symptoms of mechanical instability, surgical decompression or stabilization remains the preferred initial approach to spine metastasis management (18, 19). The improvements in expected rates of tumor control of SBRT because of dose escalation techniques have been used to justify minimizing the extent of surgical decompression to limit potentially adverse effects of surgery. Specifically, the focus of complete circumferential surgical decompression is shifting to a less invasive approach known as separation surgery, which focuses more on surgical decompression of the spinal cord/thecal sac and less on surgical cytoreduction. Surgery is then followed by postoperative SBRT (20–22). Similarly, for patients with spine metastasis presenting with mechanical instability, some centers have reported use of vertebroplasty or kyphoplasty plus SBRT as an alternative to complete surgical fixation (23, 24). By reducing the extent of surgery, these combined approaches integrating SBRT may facilitate postoperative recovery and minimize interruptions in systemic therapy in select patients (25).
Decompressive SBRT
For patients with epidural tumors, conventional options have included surgical decompression plus external beam radiation therapy versus radiation 281therapy alone, with the landmark trial of Patchell et al. setting a historic precedent favoring combined surgery and radiation therapy (7). The potent short-term palliation achieved with external beam radiation therapy alone gave rise to the hypothesis that SBRT with more rapid and durable tumor control may be used as an alternative to surgical decompression for certain patients with epidural compression (26); this approach potentially reduces the morbidity, recovery, and interruptions in systemic therapy associated with surgical decompression. This hypothesis was confirmed in a recent phase II trial by Ryu et al. with 62 patients with malignant epidural compression, showing an 80% epidural tumor response at 2 months with increased thecal sac decompression from 55% to 76% by SBRT (27). This trial presents a challenge to the conventional dogma of surgical decompression as the standard and supports the concept that patients with minimal neurological deficits can achieve effective decompression with SBRT while reserving surgical decompression for patients with rapidly progressing overt neurological deficits. For example, comparing the results from Patchell et al. and Ryu et al. (albeit with the caveat of differences in patient selection) demonstrates relatively similar ambulatory rates across groups at 84% versus 81% for all patients, 94% versus 88% rate of retaining ambulation in previously ambulatory patients, and 62% versus 59% for non-ambulatory patients becoming ambulatory (7, 27).
SBRT for Primary Spine Tumors
Benign intradural extramedullary spinal tumors such as meningioma, neurofibroma, and schwannoma are most often treated with open surgical resection. However, surgical resection carries some morbidity. Moreover, factors such as tumor location and medical comorbidities may obviate surgical resection. Building on the paradigm of effective nonoperative management for similar benign primary intracranial tumors with stereotactic radiosurgery (SRS) as an alternative to surgical resection, spine SBRT is emerging as an attractive nonoperative option for selected patients with benign primary spine tumors. A number of single institutional series have been published supporting the potential role of primary SBRT as an alternative to surgical resection in terms of both symptom relief and tumor control (28–30). However, unlike malignant spine tumors, in which life expectancy at best is typically measured over a few years, patients with benign spine tumors generally have long life expectancy, raising concerns of the potential for delayed radiation-induced myelopathy. To date, these reports have been rare and this growing body of literature promises to challenge the conventional treatment paradigms of benign spine tumors with continued follow-up (31).
IMAGING AND TARGET DELINEATION
Radiation therapy is an established treatment for painful vertebral metastases. SBRT is rapidly gaining an important role as a palliative treatment both in patients with painful disease and in patients with oligometastatic disease. Retrospective and prospective studies have shown high rates of response (pain control in 70%–90% of the patients) with very low risk of toxicity (2, 11, 32, 33).
However, clinical practice reviews of image-guided SRS (34–36) since 2011 have shown significant variability in all steps of planning and delivery, despite use of a similar platform (Elekta consortium). Therefore, a need for treatment standardization is essential.
Imaging for Assessment of Patients With Metastatic Disease Considered for SBRT
Initial assessment of patients with spinal metastases should evaluate for neural element compression, pathological fractures, bone density, and skeletal disease burden (37). Although patients with spinal metastases usually undergo plain x-rays and bone scans as first imaging modalities, their utility in the assessment of patients considered for spine SBRT is limited. These patients are usually assessed using CT, MRI of the spine, and/or PET-CT. Radiographic evaluation should be performed within 1 month prior to SBRT.
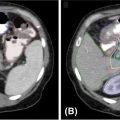
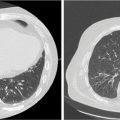
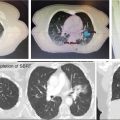
1. The gold standard for imaging of vertebral metastases is a MRI of the spine with and without IV contrast. As concurrent nonadjacent bone metastases are common, MRI of the entire spine can be helpful to assess the full extent of spinal column metastatic disease and the degree of epidural extension or paravertebral involvement. MRI is also useful in contouring both the target and the spinal cord for SBRT (however, for the latter purpose, a dedicated planning MRI in treatment position is recommended). The most useful MRI sequences for spine metastases are T1–T2-weighted images ± contrast and fat-suppressed short tau inversion recovery (STIR) sequences. Both axial and sagittal images are used (see Figure 18.1):
• T1-weighted imaging is used to identify bone pathological lesion. On this sequence, the fluid appears dark and the fat bright. Pathological bone lesions are associated with increased water content and will therefore appear hypointense (dark) on T1; such lesions typically enhance following gadolinium administration.
• T2-weighted imaging is useful in identifying the spinal cord and cauda equina as well as neural element compression, but can sometimes mask epidural involvement. Both fluids and fat generally appear bright on this sequence.
• Gadolinium-enhanced STIR imaging is characterized by suppression of the fat, which appears dark, whereas the cerebral spinal fluids (CSFs) and pathological processes generally appear bright. Therefore, this sequence may be very useful in identifying the degree of epidural and paravertebral involvement by tumor.
MRI allows identification of the degree of involvement of the spinal cord, enabling selection of patients who would benefit from SBRT alone versus those for whom a surgical decompression followed by SBRT is more appropriate (in addition to neurological assessment). Epidural spinal cord compression is a significant predictor of failure post-SBRT and the most common location of the failure. Bilsky et al. (38) described four patterns of epidural involvement and/or cord compression, from 0 (epidural extension without thecal sac deformation) to grade 3 (complete effacement of the CSF and spinal cord compression). The degree of epidural spinal cord compression is best assessed on T2 images.
2. CT scan is useful in assessing the degree of bone destruction, spinal mechanical stability, and whether the bone or the tumor itself is causing spinal cord compression. In the postsurgical setting with metal instrumentation of the vertebra, imaging can be subject to streak artifact, rendering the imaging less sensitive.
3. CT myelography is an invasive technique that consists of injecting contrast in the spinal subarachnoidal space to identify the spinal cord and exiting nerve roots. This technique can be quite useful when MRI is contraindicated or in the postoperative setting, especially when imaging artifact from instrumentation prevents identification of the spinal cord. In addition, block of dye flow on CT myelography is an excellent way to demonstrate neural element compression.
4. PET-CT can identify both the sites of bony involvement and the extent of extravertebral systemic disease. In patients with lytic metastases or mixed lytic–sclerotic metastases, fluorodeoxyglucose (FDG)-PET can differentiate between osteoporotic or traumatic fractures versus the malignant ones, using a SUVmax cutoff of 2 (39); however, it is less reliable in sclerotic lesions, characterized by a low SUVmax. If used for target delineation, care to the quality of coregistration with CT planning and/or MRI should be taken to ensure accurate targeting.

FIGURE 18.1 Identification of the metastatic lesions on MRI sequences. Example of delineation of GTV and CTV using registered MRI-CT. In this case, dedicated MRI planning (in treatment position) and CT planning were registered.
CTV, clinical target volume; GTV, gross tumor volume; STIR, short tau inversion recovery.
Imaging for Target and OAR Delineation
Volumetric thin-sliced CT and MRI are used for target delineation. Dedicated planning FDG-PET is used by some institutions.
• CT simulation should be acquired with slice thickness of 1 to 2 mm. CT remains the reference imaging modality for planning. It should extend 10 cm above and below the target.
• Dedicated MRI spine with 1- to 2-mm slice thickness is required for accurate delineation of the target and spinal cord. Although studies have shown agreement in the type of imaging used for SBRT, the type of MRI sequences is different between institutions. However, most of the studies report use of T1 and T2 sequences, ± contrast (34). Although the diagnostic MRI can be used, we recommend dedicated spine MRI in treatment position, incorporating the target and one vertebra above and below. Whenever possible, high-resolution images should be used.
• Automatic registration of CT and MRI is used; however, manual adjustments are usually necessary (34).
Target Delineation SBRT Alone
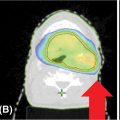
• Spinal metastases are usually enhancing, appearing bright on T2/STIR sequences and dark on T1-weighted images. Studies assessing the pattern of failure after SRS on the basis of the treated target volume indicate that treatment of the visible tumor only (gross tumor volume [GTV]) results in higher rates of failure, less pain control, and higher rates of retreatment (25, 40–43). Therefore, the current recommendation is to avoid treatment of the GTV only. There is considerable variability in the literature in target delineation and margins used to generate planning target volume (PTV); however, in 2012, the International Spine Radiosurgery Consortium (44) elaborated guidelines for contouring on the basis of the location of the metastasis within the vertebral body (see Figure 18.2):
• The GTV should include the areas of tumor involvement on the MRI and/or CT, including the epidural and paravertebral extension.
• The clinical target volume (CTV) should include the GTV and the areas of possible microscopic extension.
• The most significant variation is encountered in the definition of margins used to generate the PTV: some institutions use no margins, and in this case the CTV = PTV. Other institutions use margins between 1 and 3 mm around the CTV (PTV = CTV + 1–3 mm). If margins are used to generate the PTV, the expansion should not be isotropic; posterior expansion is usually 0 to 1 mm, allowing exclusion of the spinal cord (the most sensitive organ at risk for spine SBRT).
Target Delineation for Postoperative SBRT Spinal Metastases
Patients with malignant epidural spinal cord compression (MESCC), frank instability of the vertebral column, or impending instability generally benefit from surgery first, followed by postoperative radiation therapy. Currently, the role of surgery is circumferential decompression of the MESCC, using minimally invasive or conventional surgical techniques, facilitating the shortest possible recovery time for the patients. In this setting, SBRT is increasingly used to achieve local control. Multiple large studies have shown high rates of local control in this setting, with risk of local failure usually below 20% (22, 45). In addition, progression of the disease posttreatment is rare in the adjacent vertebral bodies, and only affected vertebrae should be treated. The most common site of failure remains the epidural space, where progression can be associated with neurological deterioration, need for reoperation, and decreased quality of life. Therefore, whenever possible, care should be taken to avoid underdosing the epidural disease, as this would be associated with geographic miss and higher risk of local failure. Separation surgery increases the distance between the epidural disease and the spinal cord and is therefore helpful in allowing better dose distribution around the epidural disease and minimizing the risk of geographic miss.
284
FIGURE 18.2 CTV concept for spine SBRT.
CTV, clinical target volume; GTV, gross tumor volume; PTV, planning target volume; SBRT, stereotactic body radiation therapy.
Source: International Spine Radiosurgery Consortium Consensus Guidelines for Target Volume Definition in Spinal Stereotactic Radiosurgery.
In the postoperative setting, recently published consensus guidelines (46) are also available to guide the contouring of GTV, CTV, and PTV. These guidelines are based on the analysis of agreement of contouring between nine radiation oncologists and one neurosurgeon with expertise in spinal SBRT. On the basis of these guidelines:
• The target delineation is based on preoperative and postoperative MRI, as well as CT simulation (same technical specifications as mentioned earlier, high-resolution, thin-slice imaging).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree