Study investigator
Number
Female (%)
Age
LM
LAD (%)
LCX (%)
RCA (%)
STEMI (%)
NSTEMI/USA (%)
Thrombolysis (%)
Medical (%)
PCI (%)
CABG (%)
Romero-Rodriguez et al. [8]
19
79
47.7
0
63
26
11
68.4
31.6
16
58
26
0
Vanzetto et al. [9]
23
74
46.3
13 %
52
22
13
60.0
40.0
0
43
35
22
Mortensen et al. [10]
22
82
48.7
9 %
86
5
14
72.0
28.0
0
32
59
9
Tweet et al. [7]
87
82
43
9 %
71
18
31
49.4
50.6
13
36
45
5
Alfonso et al. [5]
45
58
51
N/A
53
16
29
40.0
60.0
0
69
29
20
Saw et al. [4]
50
98
51
N/A
40
34
32
30
70
0
82
14
4
The true incidence is hard to ascertain and the reported incidence varies significantly. A review by Giacoppo et al. [11] showed an incidence of between 0.03 and 1.1 %. It should however be noted the diagnostic means varied widely in the numerous series included with varying denominator. An interesting recent series by Nishiguchi et al. [12] utilized OCT for diagnosis and this saw a 4 % incidence amongst acute coronary syndrome presentations, the highest incidence reported in the literature. It is therefore likely if coronary angiography or even intravascular ultrasound were the only modality used for diagnosis a significant portion of SCAD patients will be missed.
Young females are disproportionately represented in SCAD (see Table 8.1). Almost all cases presented with acute coronary syndrome, and possibly a significant portion which culminated in sudden cardiac death were never captured further adding to the common perception of underdiagnosis. Temporally there has also been a strong association of SCAD with peripartum or postpartum state [11], thought to be related to the impact of hormonal variation on arterial wall integrity.
Most SCAD patients had fewer cardiovascular risk factors than conventional acute coronary syndrome patients. All coronary branches have been reported to be affected by SCAD as had reports of multivessel involvement in the same index presentation. Recent observational data [5, 7] suggested a favourable outcome with a conservative approach, particularly in the presence of adequate or normal coronary blood flow in the affected artery; and that percutaneous coronary interventions are not infrequently unsuccessful even in experienced centers, requiring almost always more than one stent [7]. In the largest single center series by Tweet et al. [7], the 10-year recurrence rate was estimated at 29.4 %.
The recognition of such clinical factors is very important as SCAD occurs with such infrequency unless one is mindful of such clinical associations, SCAD can often be missed particularly when an angiographic radiolucent flap is not present.
8.4 Coronary Angiography
Traditionally it is recognized that coronary angiography grossly under diagnoses SCAD. Whilst this is not particularly true when a dissection or intimal flap is clearly visible on angiography, this occurs most particularly in SCAD which has resulted in diffuse stenosis with an intramural hematoma without a flap. There are many case reports in the literature where SCAD was “invisible” on angiography and the diagnosis was elucidated only on intravascular imaging.
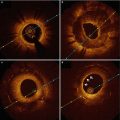
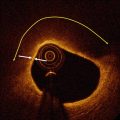
An angiographic classification for SCAD has recently been proposed by Saw [13], with three types of angiographic subtypes. Type 1 is the stereotypical angiographic appearance with a radiolucent flap (Fig 8.1a). Type 2 involves usually the mid to distal segments with often an abrupt change in vessel calibre, often extending into the terminal segment (Fig. 8.1b). Type 3 describes a focal segment of “atherosclerotic” lesion, usually in a localized segment of an artery, with compromise of side branches but usually otherwise no atherosclerotic lesion in other segments (Fig. 8.1c).
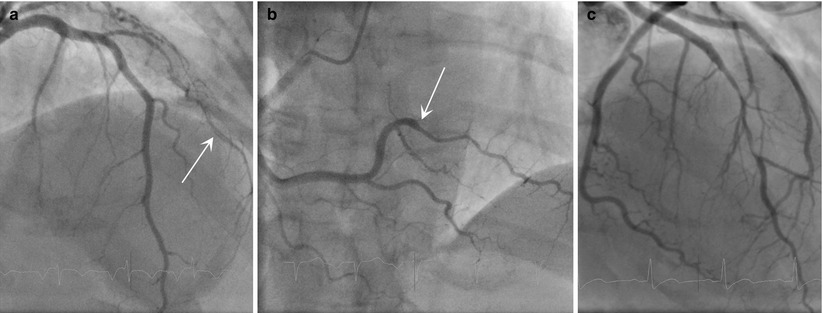

Fig. 8.1
Angiographic classification of SCAD. (a, b) Multi-vessel involvement in the same patient. (a) Radiolucent dissection flap (arrow) in the diagonal artery; (b) abrupt change in calibre (arrow) affecting the posterolateral branch. (c) Atherosclerotic type lesion affecting the mid LAD. Note unusual diffuse side branch compromise
Most conventional angiography series tend to suggest an under-diagnosis when there is a low index of suspicion amongst angiographers. However, in an unusual twist, when OCT was systemically employed for angiographically suspected SCAD lesions [14], some cases transpired to be intracoronary thrombus manifesting as filling defect, even diffuse atherosclerotic lesions. In short, when a radiolucent dissection flap is seen angiographically, the diagnosis of SCAD is reasonably specific; in the absence of a dissection flap, angiography alone is not sufficiently sensitive enough to rule out SCAD, particularly the “diffuse stenosis” subtype caused by intramural hematoma mimicking atherosclerosis (Table 8.2).
Table 8.2
Characteristics of angiography vs. IVUS vs. OCT
Coronary angiography | IVUS | OCT | |
|---|---|---|---|
Diagnosis | + | ++ | +++ |
Radiolucent intimal flap | +++ | ++ | +++ |
Microscopic intimal flap | − | + | +++ |
Diffuse intramural hematoma/stenosis | − | ++ | +++ |
Longitudinal extent of hematoma | + | +++ | +++ |
Intimal flap thickness | − | − | +++ |
Overlying thrombus | − | + | +++ |
Red vs. white thrombus | − | − | +++ |
Side branch involvement | ++ | ++ | ++ |
Associated atherosclerotic plaque | + | ++ | +++ |
Intramural hematoma characterization | − | +++ | + |
Image quality with thrombus | − | +++ | + |
Depth of penetration | N/A | +++ | + |
Large vessel assessment | +++ | +++ | ++ |
Percutaneous intervention | |||
Reference diameter/external lamina | + | +++ | + |
Entry and Exit ports | − | + | +++ |
Residual stenosis/hematoma | − | ++ | +++ |
Trapped hematoma/complication assessment | + | ++ | +++ |
8.5 Intravascular Ultrasound
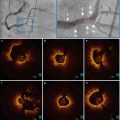
The appearance of SCAD on IVUS was first reported by Maehara et al. [15] in 2002. These IVUS studies were important in establishing the two cardinal and pathognomonic features for SCAD on intravascular imaging: intimal dissection flap and intramural hematoma. As originally suggested from autopsy studies, spontaneous intramural hematoma was the first diagnostic feature for SCAD. The finding of an intimal flap progressed the understanding of SCAD, and raised several pathogenetic mechanism. One, a spontaneous separation developed between the arterial layers, allowing the development of a false lumen, eventually leading to the dual lumen. Second, a hematoma occurred spontaneously, with the expanding hematoma eventually rupturing into true lumen causing an intimal tear from the pressurized cavity.
The enhanced resolution of OCT over IVUS has now meant however that OCT may be the intracoronary imaging of choice in SCAD, although a definitive comparison between IVUS and OCT in SCAD is yet to be performed. The first comparison between the two modalities was reported by Poon et al. [16], who suggested potential superiority of OCT over IVUS. A subsequent eight patient series by Paulo et al. [17] demonstrated that the two modalities may be complementary.
The biggest limitation to OCT in the use of SCAD is the depth of penetration, particularly in the setting of thrombus and significant intramural hematoma where the external reference diameter is difficult if not impossible to assess. Indeed, in two recent OCT SCAD series (Paulo [17] and Nishiguchi [12]), thrombus and significant residual blood render OCT uninterpretable. In Paulo’s eight patient series, IVUS provided better definition on the external layer, better assessment of the heterogeneity within the intramural hematoma, and allowed for longer segments to be imaged. A summary of the characteristics of each imaging modality is presented in Table 8.2.
8.6 Optical Coherence Tomography
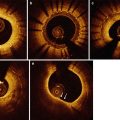
8.6.1 Diagnosis and Examination of SCAD
To date much of the data on OCT in SCAD had been accumulated from case reports and 3 larger single center case series (Table 8.3), representing no more than 50 cases of SCAD on OCT altogether. The use of OCT in the diagnosis of SCAD was first reported in 2009 by Alfonso et al. [18] and Ishibashi et al. [19] separately.
Table 8.3
OCT SCAD case series
Study | Year published | OCT | STEMI: NSTEMI | Dissection flap seen (%) | Thrombus | Atherosclerosis | Comments |
|---|---|---|---|---|---|---|---|
Alfonso et al. [14] | 2012 | 11 | 9:2 | 74 | 100 % | 27 %
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|