Primary Investigator, Country
Trial type
Treatment modality
Gleason grade included
Inclusion tests for eligibility
Follow-up tests
Objective measures
Follow-up (months)
Pow-Sang, USA
Phase I, single centre
Radiofrequency ablation
≤6
TRUS biopsy
TRUS biopsy (6 months)
Primary:
Oncological
Secondary:
Changes in QoL
6
Guazzoni,
Italy
Phase I, Single centre
Cryotherapy
≤6
TPM biopsy (≥ 12 cores)
U/K
Primary:
Safety, feasibility, tolerability
Oncological
Secondary:
Changes in QoL
60
Eggener,
USA
Phase I, Single centre
MRI-targeted laser-based thermotherapy
≤7
TRUS biopsy + MRI
−
Primary:
Adverse events
6
Trachtenberg,
Canada
Phase I, single centre
MRI-targeted laser thermal therapy
Any grade
TRUS biopsy + MRI
TRUS biopsy and MRI (4 months)
Primary:
Oncological
4
Zelefsky,
USA
Phase II, single centre
Brachytherapy
≤6
TRUS biopsy
TRUS biopsy and MRI (12 and 24 months)
Primary:
Adverse events
Secondary:
Oncological
Changes in QoL
Correlation of post-operative MRI with histology
24
Emberton,
UK
Phase II, multi-centre
HIFU
≤7
TPM biopsy + MRI
MRI and TRUS biopsy (12 months)
MRI and TPM biopsy (36 months)
Primary: Oncological
Secondary:
Functional outcomes
Short-term oncological outcomes
Changes in QoL
Health economic analysis
Correlation of imaging with histology
38
Selecting an Appropriate Patient Cohort
There are two patient cohorts that could benefit from primary focal therapy; firstly, men with low-risk disease who wish to opt for treatment over active surveillance, and secondly, men with intermediate risk disease for whom radical therapy has been offered, but who seek an alternative strategy with the aim of preserving functional status (erectile function and continence) (Fig. 19.1). These patient groups reflect the two opposing schools of thought regarding patient selection. One believes that focal therapy, as a novel treatment strategy, should only be offered to men with low-risk disease and who therefore have minimal risk of disease progression. The opposing stance is that those patients with intermediate disease are most likely to benefit from treatment if cancer control is achievable together with a lower side-effect profile.
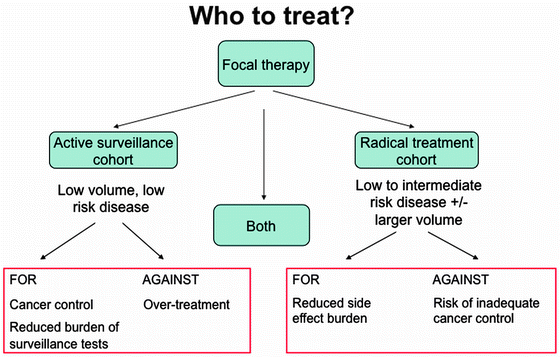

Fig. 19.1
Selection of a suitable patient cohort with localised prostate cancer for focal therapy
Focal Therapy Versus Active Surveillance
The arguments in favour of focal treatment in men with low-risk and low volume disease relate to the benefits of, firstly, halting disease progression and, secondly, reducing the psychological morbidity of living with the disease and the burden of regular PSA blood tests and biopsies.
Approximately one-third of patients undergoing active surveillance have upgrading of disease on serial transrectal ultrasound (TRUS) biopsies [16, 17]. This would suggest that at least one-third of men on active surveillance should be undergoing early active treatment. However, active surveillance relies on accurate baseline characterisation of disease burden and this upgrading may in fact be re-classification of disease in some patients due to the TRUS biopsy sampling strategy that is currently used. In other words, significant disease may have been under-sampled and under-graded on the first sampling biopsy but then sampled on the subsequent biopsy. Therefore, it is likely that a significant proportion of those men that “progress” do so not due to true cancer progression but due to the poor accuracy of diagnostic transrectal ultrasound-guided biopsies in ascertaining baseline burden. Nevertheless, low-risk disease is known to progress in some patients, and it may be these patients who would most benefit from early selective treatment. Careful evaluation of the truly “at-risk” patients is one of the main challenges we face in the treatment of prostate cancer.
In addition, the psychological morbidity of active surveillance should not be under-estimated and treatment of this group may confer significant benefit. Approximately 10% of men on active surveillance choose to have intervention despite the absence of biochemical or histological progression. However, questionnaire surveys have shown that there are conflicting findings about the anxiety levels present in these groups [18, 19]. It may be possible to alleviate patient anxiety by selectively treating cancer lesions whilst also extending the period without side effects if focal therapy were to be carried out either at diagnosis or at the time of disease progression.
The argument against focal treatment for this low-risk cohort is that any treatment may be over-treatment. Men with truly low-risk characteristics are not destined to die of prostate cancer over a 15–20-year period [20]; therefore, any intervention has a very low chance of conferring benefit and hence can potentially only confer harm. Thus, even a treatment that could offer cancer control with minimal functional impact may still carry greater morbidity than a management strategy in which two-thirds of men with low-risk disease can avoid treatment altogether.
Focal Therapy Versus Radical Treatment
The benefit of “no treatment” vs. radical treatment for localised prostate cancer remains uncertain. The Scandinavian Prostatic Cancer Group Randomised Study demonstrated a disease-specific mortality benefit of radical prostatectomy over watchful waiting (14% vs. 9%) [21]. However, the study was performed prior to the PSA screening era, in men with clinically palpable tumours and PSA levels of up to 50 ng/ml. The uptake of hormone ablation therapy was also higher in the watchful waiting cohort, with implications for comparison of survival rates between groups. A recent updated report of estimated 15-year outcomes from this study has shown a survival benefit of radical surgery that extends to the low-risk group [22]. However, the significance of this effect should be tempered in the knowledge that the limited diagnostic strategy applied at the time of this study most likely under-staged disease in a significant proportion of this cohort.
Recent advances within surgery and radiation oncology have led to significant improvements for those undergoing radical treatments for prostate cancer. Functional outcomes, recovery times and blood loss are all improved with minimal access surgery compared to traditional open techniques [23]. Radiotherapy techniques include those that are more conformal to the prostate and allow dose escalation to minimise the dose received by the peri-prostatic structures. However, the risks remain high. There is insufficient evidence to support superior cancer control with minimal access surgery compared to open radical prostatectomy, and collateral damage to the surrounding structures remains inevitable. Chronic urinary symptoms occur in one-third of men after radical surgery and in one-fifth after radiotherapy. Radical treatments also result in impotence in 30–90% of men, although modality, surgical technique and clinician expertise all impact [24]. If focal treatment can therefore offer adequate cancer control, with a lower side-effect profile, then this may become a viable standard care option for men with intermediate risk disease as an alternative to radical treatment.
A focal therapy trial could feasibly include both active surveillance and radical treatment patient cohorts, incorporating treatment of low-risk, low volume disease through to intermediate to higher risk disease. Most current or recent trials evaluating primary focal treatments select the low-risk cohort, mainly limited to treating Gleason 6 disease. A smaller number of trials include Gleason 7 disease (both dominant Gleason patterns 3 and 4). If the ablative techniques adopted for focal treatment have demonstrated adequate cancer kill, there is a good argument to broaden the inclusion criteria to those with intermediate disease in order that these men might avoid radical therapies, for the reasons described above.
Techniques for Pre-Operative Localisation and Characterisation of Disease
Imaging
(a)
Ultrasound techniques
A number of novel ultrasound techniques are now available, in addition to conventional grey-scale ultrasound. These include colour Doppler ultrasound, contrast-enhanced ultrasound (CEUS), elastography and HistoScanning™. The potential of these techniques for detecting and localising clinically significant prostate cancer looks promising [25], with significant superiority compared to standard grey-scale ultrasound. If any technique is found to have sufficient reliability and accuracy, ultrasound could be adopted as a diagnostic tool. This would have significant implications for routine clinical practice, with easy adoption within the outpatient clinic setting.
Histoscanning™ is a technique that relies on the differing backscatter produced by tissue of altered morphology, i.e. tumours, compared with normal tissue. Retrospective analyses using radical prostatectomy specimens as the reference standard have demonstrated that Histoscanning™ can reliably detect clinically significant lesions of at least 0.5 cc in volume [26, 27]. Elastography, a method that assumes that malignant tissues have different elastic properties to benign tissue, has demonstrated sensitivities of around 85%, with improved detection of high-grade disease [28]. In addition, some modalities are being evaluated for use within the context of focal therapy and for monitoring treatment effect. For example, CEUS, which relies on alterations in microvascularity with the use of microbubble contrast agents to detect disease, has been used for monitoring ablative lesion formation [29].
(b)
MRI
MRI has conventionally been used to stage disease, following a biopsy diagnosis of prostate cancer. However, developments in MRI technology and techniques have led to improved images, with the potential to apply the test earlier in the prostate cancer pathway; in other words, to detect and localise disease. This is made possible by the integration of functional images (diffusion-weighted and dynamic contrast-enhanced MRI, and MR spectroscopy) with conventional anatomical sequences (T1-weighted and T2-weighted), as so-called multi-parametric MRI. Multi-parametric MRI has demonstrated high accuracy rates for the detection and localisation of disease [30]. There is also increasing evidence that the technique can provide information on the grade and volume of a cancer lesion in the prostate [31, 32], or detect progression of disease [33].
Biopsy Techniques
TRUS biopsy, the current standard diagnostic technique, is unlikely to be sufficiently accurate for focal therapy planning. Despite an increase in the standard diagnostic protocol number of cores from 6, through 8 and 10, to the current standard of 12 cores, anterior disease and significant transitional zone disease can still be missed [34], and disease burden under-estimated. Furthermore, focal therapy relies on accurate localisation of disease, for which TRUS biopsy also under-performs. The risk of under-staging both tissue to be treated and tissue that is preserved is therefore significant with this strategy, and is likely to be reflected by suboptimal cancer control at follow-up.
The alternative biopsy technique currently available is transperineal template mapping biopsy. Biopsy cores are taken at 5 mm intervals via a brachytherapy grid. The technique is usually performed under general anaesthetic. This technique has demonstrated improved detection rates of cancer compared to TRUS biopsy [35].
The ability to visualise some cancers with imaging raises the potential for targeted biopsy sampling of suspicious lesions. An increasing number of recent studies demonstrate improved detection of clinically significant cancers using MR-targeted biopsy techniques, compared with standard TRUS sampling [36].
Integration of Diagnostic Techniques into Focal Therapy Trials
The success of focal therapy techniques is reliant on accurate detection and localisation of disease. It is therefore imperative that appropriate diagnostic investigations are selected when designing a focal therapy trial. Several focal therapy trials have included standard TRUS as the sole investigation for assessing eligibility. Other more recent studies have used transperineal biopsies. However, the distribution and density of cores that have been taken have been variable. Some limit the cores to the peripheral zone only, or to a limited number of cores throughout the prostate. Accuracy rates of cancer detection, localisation and staging will be compromised by this technique.
It is feasible to imagine that imaging and image-targeted biopsy may become the standard pathway for diagnosis of clinically significant cancer and subsequent focal treatment planning in the future. However, further data will need to be acquired to demonstrate sufficient accuracy of imaging techniques at ruling out significant disease before biopsies to the whole gland can be omitted. In the meantime, 5 mm transperineal template mapping biopsies would appear to provide the most accurate diagnostic strategy and should therefore be utilised within optimal focal therapy protocols.
There is additional opportunity to integrate imaging techniques, including multi-parametric MRI and novel ultrasound modalities, within trial protocols with prospective evaluation of their ability to detect and localise disease, using 5 mm transperineal prostate mapping biopsies as the reference standard. In order that the outputs of these imaging techniques can be formally assessed, a standardised reporting structure is critical. Reports need to be delivered in such a way that comparisons can be made between the imaging and histopathology. This is most easily achievable if the prostate is divided into sectors, with scores given for the likelihood of cancer in each. Most research studies evaluating multi-parametric MRI are now applying a 5-point Likert-type scale to report for the presence of cancer in different predefined regions of the prostate. Some groups have sought to standardise the conduct and delivery of these reports with the aim of improving outputs and comparisons [37]. Other new imaging techniques may also benefit from a similar reporting structure. In addition, adoption of these standardised imaging reports within focal therapy trials may assist in the interpretation of pre-operative and post-operative images, correlation with biopsy and other imaging platform results, and comparisons between other trials or centres.
Selecting the Disease to Treat—Cancer Cure or Cancer Control?
Focal therapy trials provide us with the opportunity of addressing two alternative strategies. Firstly, cancer cure, and secondly, cancer control. The first strategy implies treatment of all identifiable disease within a focal defined area. The second addresses the notion of treating the “index lesion,” i.e. that disease most likely to confer harm if left untreated, with ongoing surveillance of untreated clinically low-risk disease.
Up to approximately 40% of patients have been found to have either unifocal or unilateral disease [38–40]. These patients could undergo a hemi-ablation, or a more targeted focal treatment. However, for the majority, multi-focality exists throughout the gland. There is usually a dominant or index lesion of maximum volume with co-existing multiple smaller lesions. The smaller lesions usually measure less than 0.5 cm3 in volume, in contrast to a much larger average volume of the index lesion (4.3 cm3 in Stamey’s series of radical prostatectomy specimens) [41]. In addition, the index lesion also tends to confer the maximum Gleason grade [42]. There is evidence from molecular genetic studies that metastases originate from a single clone of clinically significant disease, with the suggestion (although with no definitive molecular evidence at present) that the original disease is from the index lesion [43]. This would support the notion of offering disease control by focally treating the index lesion, as long as cancer burden had been accurately assessed and localised from pre-treatment diagnostic investigations [44].
Focal therapy trials therefore have the potential for evaluating a treatment strategy that has not been formally explored before. A protocol that treats all identifiable disease, e.g. with a hemi-ablation of unilateral disease, aims to offer cancer cure, whereas a protocol that offers index lesion treatment and surveillance of remaining clinically insignificant disease offers cancer control. Some current trial protocols allow both treatment options to co-exist, with an upper threshold for untreated clinically insignificant disease of ≤3 mm Gleason 3+3 disease. It could be argued that definitive knowledge of whether index lesions drive disease progression could only be answered within a clinical trial such as this, whereby untreated clinically insignificant disease is monitored and any disease progression detected and treated early.
Selecting a Treatment Protocol
An international consensus expert panel recently defined focal therapy as ”a type of treatment that aims to eradicate known cancer within the prostate and at the same time preserve uninvolved prostatic tissue with the aim of preserving genitourinary function” [45]. This definition covers a range of protocols that could be incorporated into a focal therapy programme, with different thresholds for treatment volume. For example, the term “focal” may range from treatment of the lesion itself with a minimal margin, through to a subtotal ablation of the prostate allowing a more generous margin. Both have the potential to reduce side effect burden, as long as at least one neurovascular bundle is spared. The tighter the margin, the lower the volume of healthy tissue that would be unnecessarily treated. However, this strategy also carries a greater risk of under-treatment unless tumour locality, size and total peripheral extent can be highly accurately assessed. Image-directed treatment has the potential to permit this strategy. However, the majority of treatment modalities in use, such as cryotherapy and HIFU, currently rely on a cognitive correlation of tumour position from biopsies and pre-operative imaging for treatment planning. Registration techniques that can merge contoured MR visible lesions onto ultrasound platforms are currently under development. If successful, this technique is likely to permit greater precision of truly focal treatments.
Another consideration in setting a treatment protocol is standardisation of outputs. Hemi-ablation can be easily adopted for all focal modalities. The urethra acts as a convenient anatomical marker at the mid-point of the gland on a transverse view. Left or right hemi-gland treatments can thus be planned either side of this, or even the anterior or posterior hemi-glands. However, treatment volumes and protocols usually require tailoring to the treatment modality that is being used. Some treatments, such as cryotherapy and HIFU, are amenable to ablation of relatively large tissue volumes such as a hemi-gland. However, other treatments such as laser may be better suited to treatment of smaller tissue volumes, particularly when they demand time-consuming use of the MR scanner, and where treatment can be more accurately focused according to visual feedback from imaging. Standardisation of treatment protocols across modalities may therefore be difficult to achieve.
Another potential adaptation within a treatment plan is that of targeting the majority of the “dose” to the cancer, with a lower dose to surrounding normal prostatic tissue. Thus, a patient could undergo a hemi-ablation of the ipsilateral lesion at a therapeutic dose, with a lower dose given contra-laterally. This strategy may be preferable to those clinicians concerned with the notion of leaving clinically insignificant disease fully untreated. It may also potentially minimise the risk of leaving previously undetected very low volume disease behind. This idea is already being addressed by new techniques such as CyberKnife that, although not a true form of focal therapy, tailors the dose to be maximal at cancer lesions. Normal tissue receives a lower dose, with therefore presumed lower toxicity overall. Figure 19.2 illustrates this.
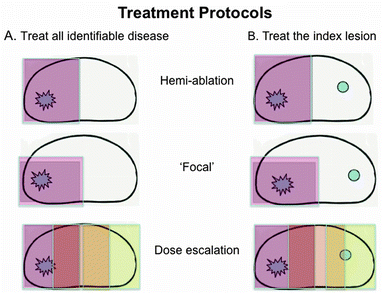

Fig. 19.2
Potential treatment protocols
Selecting Trial Outcome Measures
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree