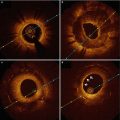
Fig. 11.1
Histology and OCT. (a) and (b) Magnified histology sections (Movat pentachrome stain) and corresponding OCT image (c, d) of a specimen of stented coronary artery with implant duration of 48 h. (a) Uncovered struts at 9 o’clock (arrowhead) and calcium deposition (arrow); (c) corresponding OCT image. (b) Thrombus deposition on top of the uncovered strut at 6 o’clock and (d) corresponding OCT image
11.6 The Role of Intravascular Imaging to Assess Stent Thrombosis
Intracoronary imaging techniques have been developed to provide complementary, more accurate measurements and details to coronary angiography in patients undergoing complex PCI. Compared with IVUS, OCT is superior in the assessment of unstable plaque features, including the presence of thrombus, rupture plaques, thin-cap fibroatheroma (TCFA) and pools of superficial macrophages [41, 42]. In addition, OCT provides superior details on the immediate results of stent implantation (vessel injury, tissue prolapse, stent malapposition and stent geometry distortion). Finally, OCT at the follow-up time is more accurate in assessing the remaining thrombus and the amount and type of tissue growing into the stent, discriminating between covered and uncovered struts, neointimal formation and newly developing atherosclerosis [42–44]. Based on these unique characteristics OCT can critically contribute to a better mechanistic understanding of the pathophysiology of ST and to the identification of surrogate markers, anticipating the risk.
11.7 OCT in Stent Thrombosis: Not All Stent Thrombosis Are Created Equal
The multi-factorial nature of ST and the high risk related to this event favors a fully informative, accurate assessment of the culprit lesion and vessel at time of acute presentation, through high resolution intravascular imaging modalities. Similarly to any acute MI, ST requires immediate thrombus removal and quick restoration of an effective TIMI flow as the goals of primary interventions. After vessel recanalization, OCT may be used for guiding a more effective thrombus removal, via mechanical and/or additional pharmacological treatments. Further, OCT may help to recognize the stent-related factors (if any) responsible for ST (malapposition, under expansion, incomplete coverage, lipid laden neointima) and to select, based upon the imaging details, the most effective treatment to avoid recurrences. Indeed, while stent under-sizing, under-expansion and other suboptimal PCI results are usually observed in the acute/subacute ST cases, the main mechanisms involved in both late/very late ST are usually linked to the presence of uncovered stent struts and/or neoatherosclerosis.
11.8 How to Perform OCT in the Acute Setting of Stent Thrombosis
The planning for imaging in a patient arriving at the hospital with possible ST should start before the patient’s arrival at the Cath Lab. If the patient was treated with stent(s) in the same hospital all clinical and procedural information (co-morbidities, clinical presentation at the index procedure, stent type and size, eventually complications at the stent implant) has to be made available to the operator before primary PCI. In patients arriving with ST and TIMI 0–1 flow grade or large filling defect, thrombus aspiration is at the frontline of treatment for reestablishing the flow and allowing subsequent histopathology analysis of the aspirated thrombotic material. In this case thrombus aspiration has to be performed until coronary flow is reestablished and in any case before the OCT imaging pullback. It is relevant to the safety of the patient and equally relevant for having high quality OCT imaging, not to proceed with the OCT scan when the vessel is occluded with no distal flow or in the presence of an extremely high thrombus burden anticipated at the angiography views. Major arrhythmias and images unreadable due to the excess of remaining thrombus are the main reasons for adopting a dedicated strategy. In the presence of a large remaining thrombus the imaging catheter can be occlusive. One possible trick to anticipating if an OCT pullback can be performed with enough quality is a quick check in angiography with a few cc of contrast, maintaining the aspiration catheter at the culprit site. If the contrast passes freely the probability that it will not be occlusive with the image catheter is high and you may proceed with the OCT pullback. A combined use of IVUS and OCT when possible is recommended, since both imaging techniques may provide complementary data on mechanisms of ST. In these cases it seems wise to start with IVUS, since no contrast flushing and no selective coronary intubation is required. IVUS may add unique information on vessel remodeling that cannot be provided by OCT. It is important to remember that OCT imaging may increase the overall amount of procedural contrast. In the presence of hemodynamic instability, frequently observed during ST, the total amount of contrast needs to be strictly monitored. When flow is reestablished the operator should take time to analyze the imaging data, understand the main causative mechanism and the possible contributing factors and to tailor the treatment strategy. Since the time interval between the baseline assessment and the final post-intervention pullbacks can be wide, the OCT catheter should be maintained wet in saline solution to avoid fibrin deposition on the imaging element and contrast medium crystallization.
11.9 Information Provided by OCT
11.9.1 Thrombus Assessment
OCT is able to assess the presence and type of thrombus and the effectiveness of coronary thrombus removal through mechanical and or pharmacological interventions. With OCT, intracoronary thrombus is identified as any abnormal mass protruding into the lumen, with a sharp gap in the underlying tissue, signal backscattering and various degrees of attenuation. White thrombus is characterized by homogeneous signal rich with low-backscattering attenuation, while red thrombus, that contains red blood cells and a network of fibrin, is defined by a highly backscattering and highly attenuated light signal (resembling blood). The presence of a large amount of residual thrombus may preclude a reliable assessment of strut coverage (Fig. 11.2). In these cases additional potent pharmacologic agents, such as GP IIb/IIIa receptor blockers, can be used to allow an accurate assessment of the underlying stent, and to detect possible segmental malapposition or consecutive frames with largely uncovered struts (Fig. 11.3).
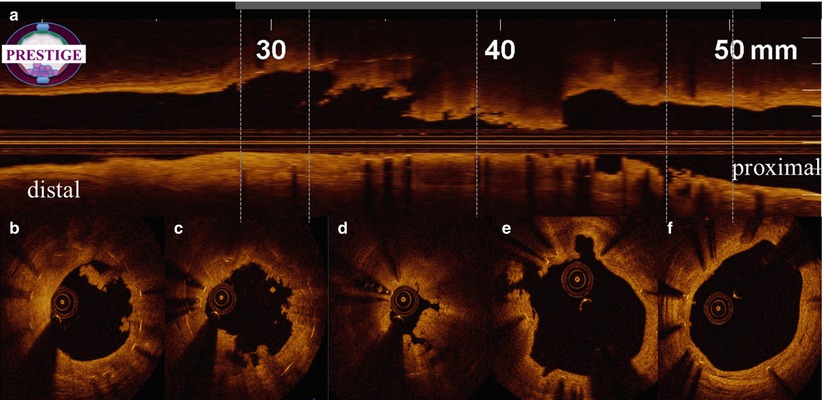
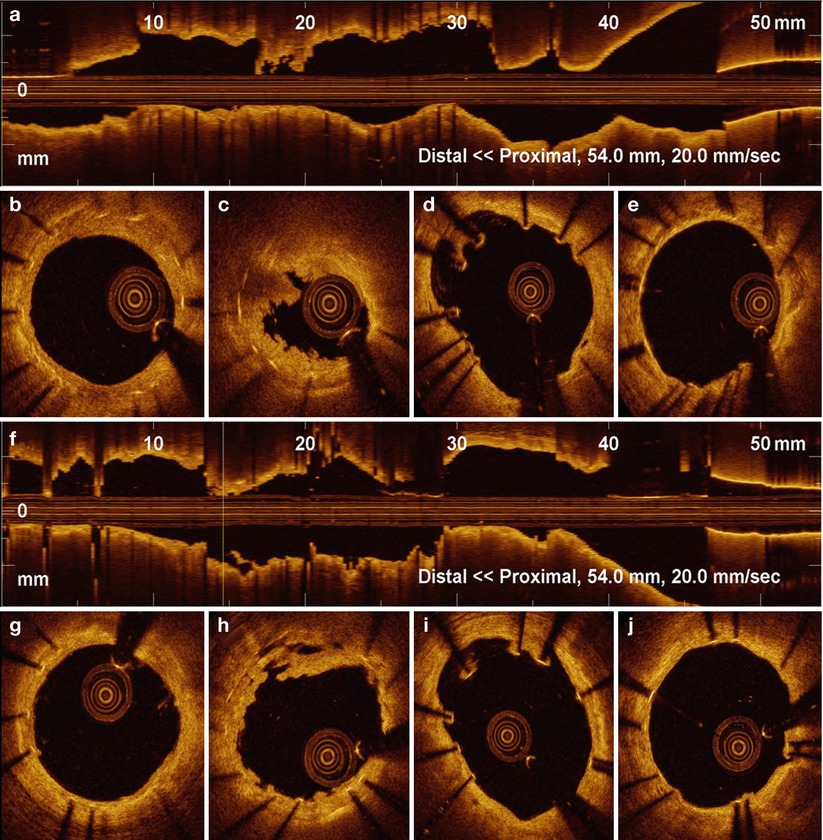

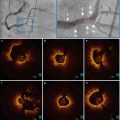
Fig. 11.2
Large amount of residual thrombus after manual thrombus aspiration. OCT longitudinal view (a) and multiple cross-sections (b–f) images during a ST case after thrombus aspiration. Large residual thrombus remained inside the stent (b, c, d). Struts are clearly visible due to shadowing. Proximal (f) and distal (b) sections indicate complete strut coverage. White thrombus mainly in (b, c), mixed thrombus in (d)

Fig. 11.3
OCT in Late Stent Thrombosis, Immediately After Thrombus Aspiration. OCT Longitudinal view (a) and multiple cross-sections (b–e) images obtained in very late stent thrombosis, immediately after manual thrombus aspiration. Large residual thrombus is observed inside the stent, dominantly white in signal characteristics (c). Struts malapposition can be observed (d). Same vessel after intracoronary injection of GP IIb/IIIa inhibitors. A substantial reduction of the remaining thrombus can be recognized in long (f) and cross-sectional (h) images. Incompleteley apposed stent struts can be identified in the cross sections proximal to thrombus burden (d, i). Healthy looking distal (g) and proximal (j) stent segment
11.9.2 Inadequate Stent Implantation
Inadequate stent implantation can be due to a mismatch between lumen dimensions and the selected stent size, or be the consequence of stent under expansion in spite of an adequate stent/artery ratio. Providing automatic lumen profile measurements, novel OCT systems allow for the online detection of inadequate stent implantation.
In a small-scale OCT study evaluating patients with subacute ST [45], a smaller minimal stent area was observed in these patients compared to the uneventful controls, with minimum stent area preferentially located at the thrombus site, indicating stent under expansion as a possible mechanism of early ST. In addition, the percentage of both uncovered and malapposed struts was significantly higher at the thrombus site compared with their respective control patients.
Illustrative Case 1
Stent Under-Expansion as Primary Cause of Late Stent Thrombosis
A 60 years-old patient underwent a complex PCI procedure on a highly calcified right coronary artery (RCA), requiring rotational atherectomy (Fig. 11.4). Five years later the patient was admitted with diagnosis of inferior STEMI. Coronary angiography revealed an in stent thrombotic occlusion of the RCA. After wire crossing and thrombus aspiration, OCT and IVUS pullbacks were performed. IVUS showed a large amount of residual thrombus and severe stent under expansion at the culprit segment. OCT pullback confirmed stent under expansion in the absence of any uncovered/malapposed struts or abnormal neointima (Fig. 11.5). Severe under expansion was treated with larger balloons and a short EES implanted at high pressure. Final intracoronary imaging confirmed an adequate acute gain in the previously unexpanded stent segment (Fig. 11.6).
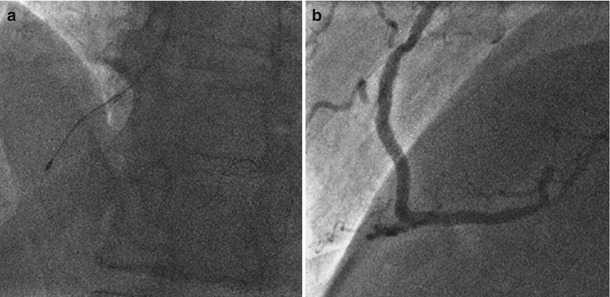
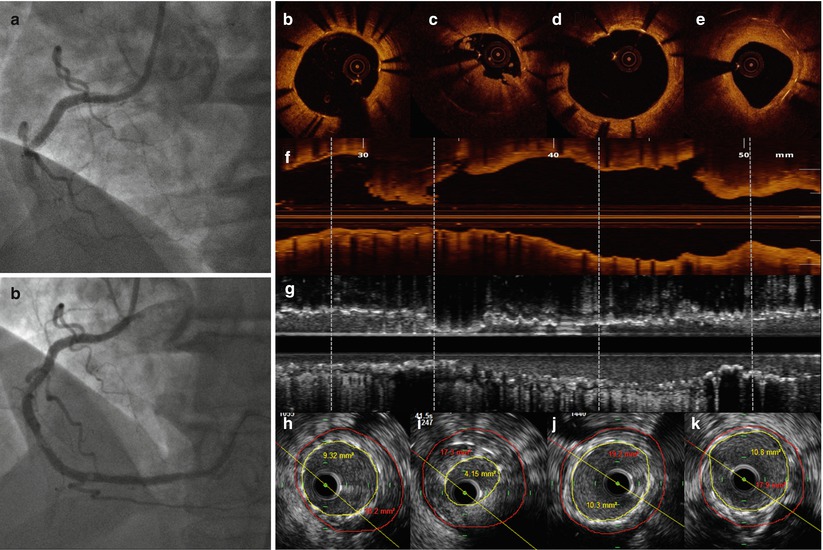
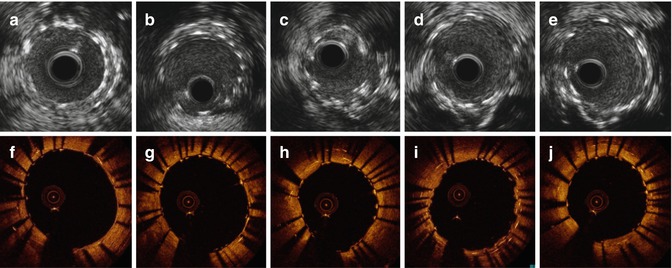

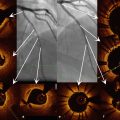
Fig. 11.4
Coronary angiography at the index procedure. (a) Rotational atherectomy at mid RCA. (b) Optimal result after DES implantation

Fig. 11.5
OCT and IVUS findings at time of very late ST. (a, b) coronary angiography showing stent thrombosis at mid RCA (a) and lumen profile after reestablishing of the vessel patency with thrombus aspiration (with small persisting filling defects). (b, d, e) OCT cross sectional views at multiple levels. Distal and proximal stent segments demonstrated complete strut coverage, no malposition and only mild tissue deposition (e). (c) OCT cross section with stent under-expansion at the site of protruding thrombus, remaining after manual aspiration. The thrombus was not detected at the corresponding IVUS section (i). (f, g) Longitudinal OCT and IVUS views. Distal (h) and proximal (j, k) to the culprit lesion (i) IVUS cross sectional images, show good stent expansion and moderate vessel remodeling

Fig. 11.6
IVUS and OCT assessment post-treatment. (a–e) IVUS and (f–j) OCT multiple cross-sectional views. Large MLA, even at site of multiple strut layers
11.9.3 Incomplete Stent Apposition
There is increasing interest in the role of incomplete stent apposition (ISA) as a causative factor of ST. ISA is normally defined as the separation of one or more stent struts from the arterial wall, with evidence of blood speckles behind the strut, in the absence of any side branch. According to the time of occurrence, ISA is labeled as acute (post-procedure), persistent (diagnosed at post-procedure and still present at follow-up), and acquired (not present at post-procedure but identified at follow-up). In the absence of any intravascular imaging assessment at the index procedure, ISA is usually classified as late incomplete stent apposition. The role of stent malapposition in the pathogenesis of late ST has been continuously debated advocated as a possible causative factor in some studies and neglected in others. Cook et al. reported an increased incidence of stent malapposition and positive vessel remodeling in patients presenting with very late ST after first generation DES, compared with uneventful controls [46]. ISA was detected in 77 % of patients with very late ST with a wide area of malapposition. Similarly, in a study conducted by Guagliumi and coll. on consecutive cases of late and very late ST assessed with OCT, stent malapposition was observed in 78 % of the all cases, with maximal area of malapposition significantly greater compared to the uneventful matched controls [43]. Recently, the “Mechanism Of Stent Thrombosis” MOST study also demonstrated a three times higher rate of malapposed struts in patients with late ST as compared to controls [45]. Late acquired ISA may essentially be due to two different mechanisms: (a) positive vessel remodeling, as a marker of vessel toxicity in response to the drug and or to the polymer; (b) resolution or reabsorption of a pre-existing soft plaque or thrombus behind the stent struts.
Illustrative Case 2
Persistent Malapposition as Causative Factor of Stent Thrombosis
Brief summary: Two everolimus-eluting-stent (EES) were implanted to treat an occluded left descending coronary artery (LAD) during an anterior STEMI. The proximal stent was bordering a small aneurysm (arrow, Fig. 11.7). After 4 years, the patient was urgently hospitalized due to a recurrent anterior STEMI. Coronary angiography showed a thrombotic occlusion at the stent entrance. After wiring the occluded vessel and an effective manual thrombus aspiration, coronary angiography displayed only a small filling defect, with irregular borders at the level of a small aneurysm (Fig. 11.8). An IVUS pullback, sequentially performed after OCT, excluded the presence of positive vessel remodeling, confirming the persistent nature of malapposition. Large malapposition associated with lack of coverage was identified as the causative mechanism in this EES ST (Fig. 11.9). To further expand the entrance of the existing stent, and promote the strut coverage in this small aneurysm, a large BMS was implanted at malapposition site and finally expanded at high pressure (Fig. 11.10).
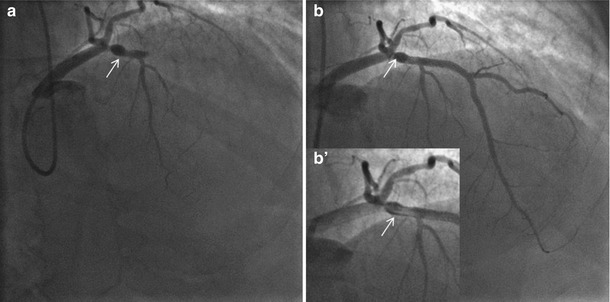
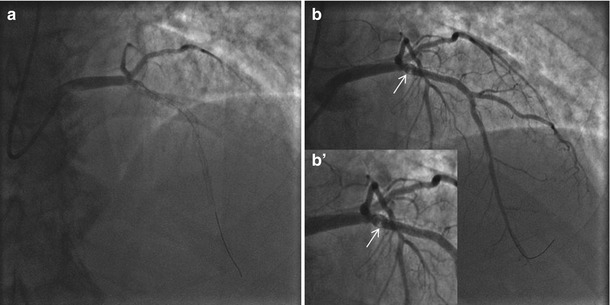
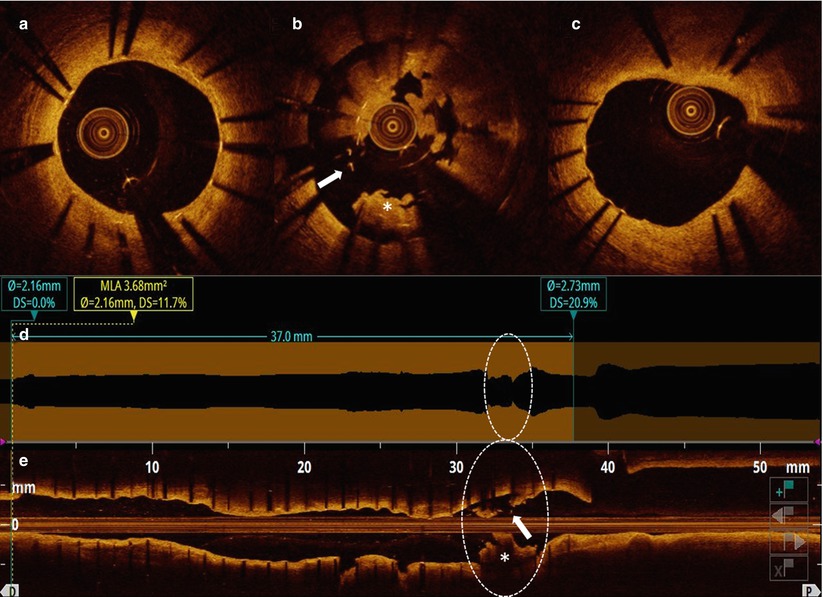
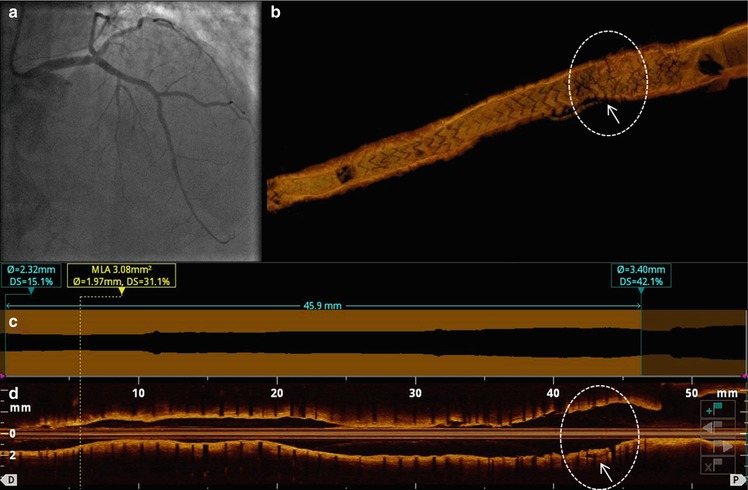

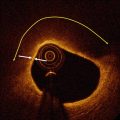
Fig. 11.7
Index primary PCI on LAD during an anterior STEMI. (a) Coronary angiography at the index procedure (primary PCI) with LAD occlusion. A small aneurysm was visible (arrow) immediately before the occlusion point. (b) Coronary angiography immediately after stent implantation with a reestablished lumen profile and effective coronary flow. (b’) Magnification at the stented segment with evidence of contrast staining bordering the small aneurysm (arrow)

Fig. 11.8
Coronary angiography performed during very late ST. (a) Coronary angiography showing an in stent thrombotic occlusion. (b) After thrombus aspiration and restored vessel patency. (b’) Magnification at proximal LAD after thrombus aspiration: persistent haziness at the level of the aneurysm located at the stent entrance (arrows)

Fig. 11.9
OCT longitudinal and cross-sectional views after thrombus aspiration. Cross-sectional views: (a, c) optimal healing and coverage of the stented segment at both distal and proximal sites. (b) Cluster of grossly malapposed and uncovered struts (arrow) with large remaining thrombus. Automatic lumen border detection (d) and long view (e) showing overall uniform and good stent expansion, except for the segment at the aneurysm level (circle)

Fig. 11.10
OCT pullback after BMS implantation at the malapposed site. Coronary angiography (a) and OCT at post-treatment. The 3D OCT reconstruction (b), automatic lumen profile (c) and longitudinal view (d) confirmed optimal stent expansion with no residual malapposition (arrow) in the segment of interest (circle)
This case shows some of the issues with stent implantation during acute myocardial infarction, when the presence of thrombus and the increased vasoconstriction make difficult the stent size selection and the identification of the proper landing zone, facilitating stent malapposition to remain undetected by angiography. In such cases a large segment of malapposed/uncovered struts may persist asymptomatic for years until a triggering factor may precipitate ST.
Finally, as demonstrated by Gutierrez-Chico and coll., the larger the acute malapposition, the greater the likelihood of persistent malapposed segments at FU and delayed healing [47].
Illustrative Case 3
Acquired Malapposition in Current Generation DES as Cause of Stent Thrombosis
A 50 year old man presented with an anterior STEMI and cardiogenic shock. The proximal LAD was then treated by implantation of a 3.5/24 mm everolimus eluting stent (Fig. 11.11). Two months later, despite an adequate DAPT, the patient experienced recurrent chest pain and was admitted with diagnosis of non ST elevation myocardial infarction (NSTEMI). Coronary angiography revealed invariable stenosis in the left circumflex artery (LCX) and a barely noticeable in stent hazy lesion in the previous stented LAD. Fractional Flow Reserve (FFR) investigation of LCX resulted in a non-ischemic value. OCT revealed uncovered and malapposed stent struts, and a luminal protruding mass combining the characteristics of both organized and fresh thrombus (Fig. 11.12). The diagnosis of late ST as the culprit lesion was therefore made. Interestingly, the MULTIPLATE analyzer testing platelet reactivity revealed sufficient platelet inhibition by ongoing therapy. Thus the hypothesis of persistent or acquired ISA, was considered as the possible substrate for this complication. Application of high pressure balloon (4.5/12 mm at 20 atm) dilatation resulted in a larger cross-section area and corrected stent apposition (Fig. 11.13).
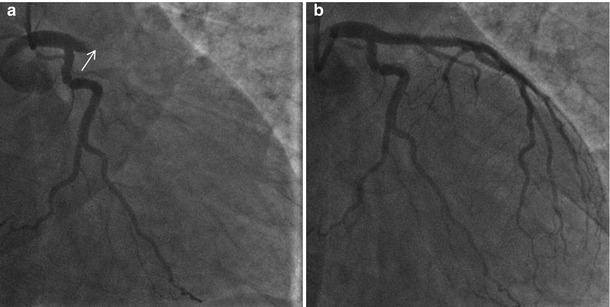
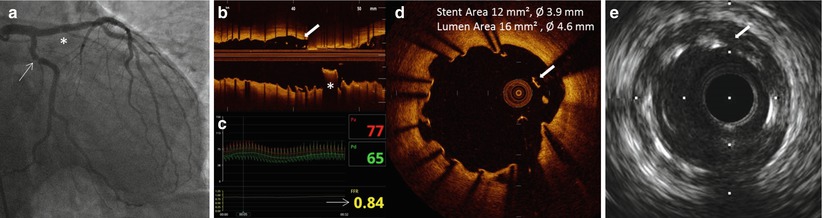
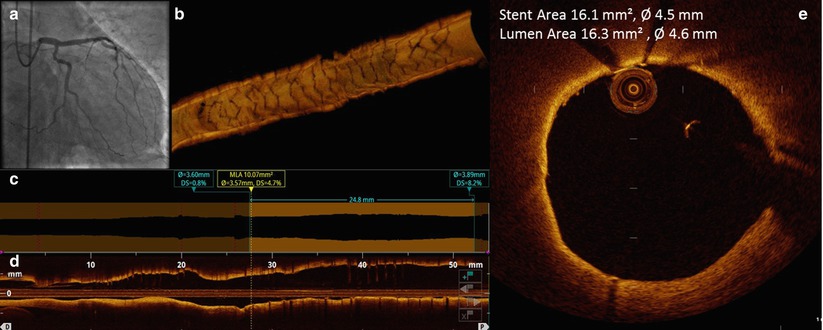

Fig. 11.11
Baseline procedure. (a) Coronary angiography during index primary-PCI showed thrombotic occlusion of left anterior descending artery (arrow). (b) Coronary angiography after stent implantation showing acceptable result

Fig. 11.12
Angiographic and OCT findings of late stent thrombosis. (a) Coronary angiography shows an area of haziness inside the EES previously implanted in the left anterior descending artery, and a tight focal stenotic lesion in mid circumflex artery (arrow). (b) Longitudinal reconstruction after thrombus aspiration revealed the thrombotic nature of the intraluminal hazy lesion. (c) FFR performed on circumflex artery was negative (d) close review of cross-section OCT reveals clusters of uncovered and malapposed struts. (e) IVUS pullback revealed absence of positive vessel remodeling and free space behind stent struts

Fig. 11.13
OCT pullback after high pressure dilatation. (a) Final coronary angiography demonstrated good angiographic results of high pressure balloon dilatation. (b) 3D OCT reconstruction showed perfect result with optimal stent strut geometry; (c, d) Lumen profile tool and longitudinal reconstruction did not revealed residual stenosis and stent edge dissection after high pressure balloon stent expansion. (e) Cross-section analysis at the proximal stent edge showed resolution of the strut malapposition
This case illustrates that secondary thrombus resolution in the contest of STEMI may cause ST due to acquired stent malapposition even in patients responsive to DAPT therapy. The use of OCT helped in the identification of mechanisms underlying ST and guided a customized treatment with a high pressure balloon dilatation. Combined use of imaging technology with platelet reactivity tests are sometimes needed to detect the mechanism underlying ST events.
ISA after stent implantation may also present with acquired in-stent aneurysm(s) formation and largely uncovered/malapposed struts. This results mainly from a toxic reaction to a drug and/or polymer creating extensive coronary wall inflammation with in stent extra-cavities formation. The segment involved has a significant damping of the optical signal (dark segmental appearance) compared to the uninvolved segments that normally have a mature neointima. These aspects were recently substantiated in an OCT study assessing different degrees of maturity on top of the stent struts, in various preclinical and clinical contexts (ex-vivo animals and humans, and in-vivo animals and humans) [48].
Illustrative Case 4
Very Late Stent Thrombosis due to Late Acquired Malapposition
Fifty-seven years old male presenting with multi-vessel coronary artery disease was treated with PCI and two PES implanted in overlap on the proximal LAD. Three years later the patient was readmitted for an anterior STEMI in cardiogenic shock complicated by cardiac arrest. Emergency angiography, under external cardiopulmonary resuscitation, documented very late ST on the proximal LAD, prompt balloon dilation immediately restored vessel patency. Extracorporeal support (ECMO) was immediately initiated. A few days later, with haemodynamic stability, an OCT pullback displayed the presence of an acquired in-stent aneurysm with extra-cavities around the entire circumference of the vessel, and largely uncovered and malapposed struts (Fig. 11.14). As treatment, a PCI with a bare metal “stent in stent implantation” was performed in an attempt to promote strut coverage and seal these extra-cavities. The patient was then discharged on the 30th day with complete left ventricular function recovery. At the 6 months OCT follow-up a complete sealing of the in-stent aneurysm with homogeneous stent coverage was observed (Fig. 11.15).
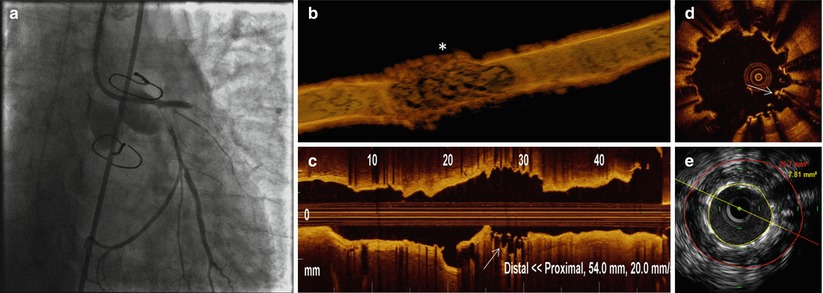

Fig. 11.14




Coronary angiography, OCT and IVUS during very late ST with cardiac arrest. (a) Coronary angiography during cardiac arrest showing thrombotic occlusion of proximal LAD and LCX. (b) 3D OCT reconstruction after primary-PCI and hemodynamic stabilization revealed a segmental in-stent aneurysm at site of overlap (*asterisk). (c, d) Long view OCT and cross section analyses showed clusters of uncovered and malapposed struts (arrow). (e) IVUS pullback confirmed large positive remodeling at the stent overlap, (arrow).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree