where n = number of treatment fractions, d = dose per fraction, and α/β is the ratio of the linear and quadratic components of the cell survival curve ; for the purposes of their study, an α/β ratio of 10 was assumed. For example, a regimen of 54 Gy in 3 fractions would have a BED of
 or 151.2. They found that increasing BED to doses >150 Gy equivalent was associated with improved survival in patients undergoing SBRT/SABR for larger (T2) tumors [58].
or 151.2. They found that increasing BED to doses >150 Gy equivalent was associated with improved survival in patients undergoing SBRT/SABR for larger (T2) tumors [58].While local control rates with SBRT/SABR in early-stage NSCLC are excellent [40, 59], distant failure is common, occurring in 20–30 % of patients in 3–5 years [40, 60–62]. Future efforts in the treatment of early-stage NSCLC will naturally include optimization of treatment delivery to safely and accurately deliver ablative doses to tumor while limiting normal tissue toxicity, but it is likely that incorporation of appropriately timed and administered cytotoxic, targeted, and immunotherapy-based treatments will be required to optimize outcomes in terms of out-of-field tumor recurrence and overall patient survival after SBRT/SABR .
Specific Issues Associated with SBRT/SABR for Targets in the Lung
Escalating the dose to the target in the lung has been shown to be effective in terms of killing the tumor cells, but the normal nearby tissues must be taken into account; tumor control does come at a price. The lung may be considered both a parallel and serial organ , in that there is some redundancy due to its paired nature and parenchymal reserve , but injury to a central structure may impair function of a large downstream volume; one aspect of this is the proximal bronchial tree . Ablative doses given to a very proximal branch of the airway could cause injury that impairs downstream function and lead to significant patient pulmonary toxicity ; additionally, large vessels run in close approximation to these large branches and could also potentially be a target for injury. In a study by Timmerman et al., building on an earlier dose-escalation study [10], 70 patients with T1-2 N0 medical inoperable NSCLC were treated with either 60 Gy in 3 fractions (for T1 disease) or 66 Gy in 3 fractions (for T2 disease); these doses were also calculated without correction for tissue inhomogeneity, and there was no restriction on tumor location. Local tumor control remained very high, 95 % at 2 years; however, on follow-up, eight patients had serious grade 3 or 4 toxicities (declining pulmonary function, pneumonia, effusion, apnea), and six patients died of possible grade 5 toxicities, including one fatal hemoptysis four infectious pneumonias , and one pericardial effusion . Tumor location was associated with severe toxicity, and this study identified that dose delivery to targets overlapping the proximal bronchial tree with a 2 cm expansion (consisting of the carina, the right and left main bronchi, the right and left upper lobe bronchi, the bronchus intermedius , the right middle lobe bronchus the lingular bronchus ;, and the right and left lower lobe bronchi) was most predictive of serious adverse effects. This area was defined as a “no-fly zone” for SBRT/SABR in the lung of very high fraction sizes (>10 Gy per fraction) [63].
Effective dose delivery for patients with “central tumors” is an area of active investigation. The RTOG recently closed RTOG Protocol 0813 , which was a Phase I/II study of SBRT/SABR for the treatment of early-stage , centrally located NSCLC in medically inoperable patients. They defined central tumors as those with any overlap with a 2 cm expansion from the previously defined proximal bronchial tree , as well as any lesions adjacent to the mediastinal or pericardial pleura. Dose was delivered in 5 fractions every other day, starting at 50 Gy in 5 fractions and escalating to 60 Gy in 5 fractions .
SBRT/SABR for Metastases to the Spine
Radiation therapy has a role in the management of both primary and metastatic lesions of the spine, although the vast preponderance of metastatic disease has led to more extensive research and clinical evaluation of treatment techniques. Metastatic disease in the spine is common, accounting for up to 70 % of all metastases to the bone and affecting up to 10 % of all cancer patients [35, 64]. Spine involvement can result in back pain (the most common presenting symptom) and deterioration in functional status and quality of life [65]. Compression or invasion of the spinal cord, cauda equina, or exiting nerve roots can lead to disabling or even life-threatening neurological symptoms [66].
Conventionally fractionated radiation therapy for spine metastases is generally a palliative therapy and may not be sufficient alone to restore and maintain neurological function; in a study by Patchell et al., patients with epidural spinal cord compression were randomized to conventional external beam radiation therapy (30 Gy in 10 fractions) alone or direct decompressive surgery followed by radiation therapy. Patients who underwent combined modality treatment had significantly improved neurological outcomes, with more patients able to ambulate after treatment (84 % vs 57 %, P = 0.001) and longer sustained ambulatory status (122 days vs 13 days, P = 0.003). A small survival benefit was also noted (126 days vs 100 days, P = 0.033) [67]. Conventional external beam therapy has been shown to achieve local control rates range less than 50 % [68–71]. Even in the postoperative setting, in a large retrospective study by Klekamp and Samii, patients receiving low-dose conventional external beam radiation therapy following surgery for spinal lesions had documented local failure as high as 58 % at 6 months, and these local failures led to neurologic deterioration in 69 % of the patients within 1 year and in 96 % of patients within 4 years [69].
Multiple studies support the hypothesis that dose escalation, particularly in terms of dose per fraction, improves the likelihood of local control in lesions metastatic to the spine [72–75]. Hartsell et al. conducted a randomized trial in which 898 patients with painful bone lesions (patients with spinal cord or cauda equina compression were excluded) were treated with either 8 Gy in 1 fraction or 30 Gy in 10 fractions. The two regimens were equivalent in terms of pain and narcotic relief at 3 months, with less acute grade 2–4 toxicity in the 8 Gy arm (10 % vs 17 %); retreatment rates were doubled in the 8 Gy arm (18 % vs 9 %), suggesting that a single high-dose fraction could provide comparable benefit to a more protracted course [76]. With advances in radiation therapy delivery, fraction sizes above 8 Gy could be delivered to spinal targets while constraining dose to the spinal cord and/or cauda equina [77]. The use of SBRT/SABR techniques with precise target delineation allows for safe delivery of radiation while limiting dose to the nearby spinal cord; techniques for defining the spinal cord vary, with some institutions preferring a CT-myelogram-defined cord immediately prior to simulation [78, 79], while other institutions define the cord on the basis of a registered and fused T1- and T2-weighted MRI, which is the method used in the current RTOG (now NRG Oncology ) 0631 protocol . A more conservative approach pursued at some institutions defines the organ at risk as the entire thecal sac or canal [80]; this approach is often used at the level of the cauda equina [74].
A Phase I/II non-dose-escalating study was performed by Chang et al. using SBRT/SABR for spinal metastasis, pattern of failure analysis. In their initial Phase I report [81], they treated 15 patients with SBRT/SABR to a goal dose of 30 Gy in 5 fractions, constraining the spinal cord to a maximum dose of 10 Gy. Five of the patients treated on the study had been previously irradiated. No neurotoxicity or grade 3–4 toxicities were observed. In the subsequent failure analysis report [82], a total of 63 patients with 74 tumors had been treated to doses of 30 Gy in 5 fractions or 27 Gy in 3 fractions; 1-year freedom from tumor progression was 84 %. Of the local recurrences, 47 % were located in the epidural space, where effective dose delivery was most constrained by the proximity of the spinal cord [81, 82]. The correlation between failure to deliver maximal dose and increased risk of failure has received attention from multiple investigators. Lovelock et al. [83] reported a study of dosimetric coverage of target lesions and found that portions of gross tumor volumes (GTV) receiving less than 15 Gy were at highest risk of failure. These deficits in GTV dosimetry were often due to constraints placed on the radiation treatment planning process in terms of the maximum dose (D max) permitted to the spinal cord.
A dose-escalation protocol initiated at Memorial Sloan Kettering Cancer Center (MSKCC) using image-guided single-fraction high-dose radiotherapy for metastatic disease established 24 Gy to the planning target volume (PTV) as an effective dose to achieve 85–95 % tumor control for spine lesions, osseous metastases, and soft-tissue/lymph node metastatic deposits (MSKCC Protocol 06-101) [77, 84]. Yamada et al. reported on 93 patients with 103 spinal metastases treated with 18–24 Gy in a single fraction. Using this regimen, 90 % overall actuarial local control was achieved at a median follow-up of 15 months; patients treated with the highest dose level of 24 Gy had superior local control (95 % vs 80 % for single-fraction treatments <24 Gy) [77].
Some tumors , such as renal carcinoma and sarcoma , have been shown to be less sensitive to fractionated radiation than other histologies and also have limited systemic treatment options. These tumor histologies provide a particularly useful model for testing the efficacy of SBRT/SABR, as local control outcomes are not confounded by competing therapies [85]. Zelefsky et al. reported on tumor control outcomes after hypofractionated and single-dose SBRT/SABR for extracranial metastases from renal cell carcinoma; of the 105 lesions treated on the study, 59 (56 %) were located in the spine. For patients who received 24 Gy in a single fraction, 3-year local progression free survival was 88 %; for patients receiving single fractions of less than 24 Gy or hypofractionated regimens of 24–30 Gy in 3–5 fractions, 3-year local progression free survival was 21 % and 17 %, respectively [75]. Folkert et al. reported on 88 patients with 120 discrete metastases from high-grade sarcoma to the spine, treated with hypofractionated or single-fraction SBRT/SABR. Local control at 12 months was 88 %, with single-fraction treatments of 24 Gy having superior outcomes (1-year local control of 91 %, compared to 84 % for hypofractionated courses of 24–26 Gy in 3–6 fractions) [73].
A currently open RTOG trial, RTOG 0631 (NCI designation NCT00922974), is comparing the relative benefit of 2 single-fraction regimens: 8 Gy in 1 fraction delivered with conventional techniques and 16–18 Gy delivered in 1 fraction using SBRT/SABR techniques. Clinical response, in terms of pain reduction at 3 months, is the primary objective of the Phase III portion of the study. Initial Phase II results have been published demonstrating the feasibility and reproducibility of the technique [86]; while local control outcomes are not a specific objective of the study, the potential exists to provide a direct comparison of objective radiographic response to low- and high-dose single-fraction regimens.
Specific Issues Associated with SBRT/SABR for Targets in the Spine
Treatment of targets in the spine can be particularly complex as the spine circumferentially encloses critical neural structures. A critical toxicity that must be taken into account with treatments affecting the spinal cord is radiation myelitis . Radiation myelopathy is defined as clinical signs and/or symptoms of sensory or motor deficits , with progressive loss of function or neuropathic pain, referable to a level of the spinal cord treated by radiation therapy and confirmed by radiographic means [87–89].
The generally accepted dose limit for the spinal cord is 45 Gy at 1.8–2.0 Gy/fraction [89]; 50 Gy is observed in otherwise healthy patients treated with curative intent where the tumor location prohibits limiting the cord to a lower dose, with an attendant 5 % risk of myelopathy at 5 years [87, 89]. For patients undergoing high-dose spinal cord radiosurgical procedures, spinal cord tolerance is defined as a cord maximal dose of 14 Gy or less than 10 Gy to 10 % volume of the spinal cord per level [77, 90]. In the event of failure, these limitations may preclude or impair the ability of radiation oncologists to offer effective salvage therapy with external beam techniques . Toxicity resulting from repeat irradiation is a subject of open investigation, with thresholds of 100–135 Gy in biologically effective dose (BED) proposed for late complications due to repeat irradiation of the spinal cord [91–93]. Outcomes were evaluated with respect to biologically effective dose (BED) [57], which is calculated according to the simplified formula:
 where n = number of treatment fractions, d = dose per fraction, and α/β is the ratio of the linear and quadratic components of the cell survival curve; for the purposes of spine irradiation, an α/β ratio of 2 may be assumed. For example, a tolerance dose of 14 Gy in 1 fraction would have a BED of
where n = number of treatment fractions, d = dose per fraction, and α/β is the ratio of the linear and quadratic components of the cell survival curve; for the purposes of spine irradiation, an α/β ratio of 2 may be assumed. For example, a tolerance dose of 14 Gy in 1 fraction would have a BED of  or 112 Gy.
or 112 Gy.

 or 112 Gy.
or 112 Gy.Preclinical data exists in swine models , as well as several published institutional experiences with multiply irradiated patients , that suggests that the tolerance of the human spinal cord to re-irradiation may be greater than currently assumed and practiced. A study by Medin et al. [94] used a swine model in which two sets of pigs underwent single-fraction SRS at a series of increasing spinal cord D max (approximately 15, 17, 19, 21, 23, and 25 Gy); one set had previously undergone irradiation of the spinal cord 1 year prior to SBRT/SABR, receiving 30 Gy in 10 fractions (BED = 75 Gy). No differences in the rates of spinal cord injury were noted in the previously irradiated swine cohort compared to the unirradiated cohort, and no neurologic injuries were noted at spinal cord D max <18.8 Gy. In humans, Katsoulakis et al. [95] studied a cohort of ten patients treated with three courses of radiation to the same site in the spine; the median spinal cord total D max BED for the cohort was 141.5 Gy BED (range 103.8–203.4 Gy BED). In this cohort, no cases of clinical radiation myelopathy were observed with a median total follow-up of 40 months from the first course of radiation and 12 months from the third course of radiation. Additionally, no MRI spinal cord signal changes were noted.
Determining the re-irradiation tolerance of the spinal cord is the objective of a prospective Phase I clinical trial investigating the use of single-fraction re-irradiation following local progression of mobile spine and sacral lesions that have previously received radiation therapy. Patients on this trial will be treated with single-fraction SBRT/SABR at three cord tolerance levels, starting with a spinal cord/cauda D max of 14 Gy, escalating to 16 and then 18 Gy (NCI designation NCT02278744).
SBRT/SABR for Primary Liver Cancer
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of cancer death [96]. Hepatocellular carcinoma most commonly arises within a background of chronic liver disease [97], and the most common risk factors for the development of HCC are alcohol use and viral infection with hepatitis B and/or hepatitis C [98]. In the United States, the incidence will continue to rise dramatically necessitating early diagnosis and definitive therapy [99]. Due to the increasing incidence of HCC, routine surveillance strategies are in place which allow for earlier detection of disease in patients at high risk [100].
The current treatment schema for patients with HCC is defined by the Barcelona Clinic Liver Cancer (BCLC) strategy. This takes into account the quantity of tumors, the size of tumors, Child-Pugh’s score, and extent of invasion [101]. Potentially curative treatment for patients with HCC can be performed with orthotopic liver transplantation (OLT) , which treats both the underlying cirrhosis as well as the malignancy. Candidacy for liver transplantation is based on patients with early-stage disease, consisting of Child-Pugh score A–B , a single nodule <5 cm or 3 nodules <3 cm, and candidacy for transplantation.
Aside from OLT, surgical resection and percutaneous ablation are the treatments which provided the highest potential of cure [100]. Percutaneous radiofrequency ablation is the treatment of choice for patients not candidates for surgical resection. During treatment, the tumor and a margin of adjacent hepatic tissue are treated with results as effective as resection for small, solitary nodules of HCC [102]. Transarterial chemoembolization is a procedure which takes advantage of the dual blood supply of the liver to deliver antineoplastics plus a gelatin sponge to arterial vasculature supplying the tumor [103]. The seminal meta-analysis of TACE versus systemic therapy found an improvement in the 2-year survival rate [104], and it is recommended for patients with BCLC intermediate-stage disease.
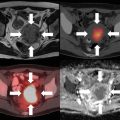
For patients with BCLC early-stage disease , SBRT/SABR can be considered as an alternative for patients not amenable to RFA due to tumor size or proximity to vessels. A substantial proportion of patients present with disease outside of transplant criteria or will progress outside of transplant criteria while on the waiting list, which necessitates the need for “bridging” therapies. It is here where modalities for downstaging or bridging can be aided by the utilization of SBRT/SABR. Furthermore, among patients with BCLC intermediate-stage disease , SBRT/SABR can be used following failure of TACE or as an alternative for TACE in patients who are not candidates for therapy. Follow-up of patients treated with SBRT/SABR with HCC includes dedicated liver imaging, ideally with MRI. There is considerable work being performed on characterizing imaging features in the cirrhotic liver post-SBRT/SABR, with Fig. 8.1 showing features of a treated lesion.


Fig. 8.1
HCC treated with SBRT . Pathognomonic arterial enhancement and venous washout seen pretreatment, which gradually resolved representing tumor response. T2-weighted imaging shows progressive evolution of edema within irradiated volume (a–e) [145]
Our commonly utilized dose regimen for patients with HCC is based on the Indiana University experience. In a Phase I feasibility trial, patients with HCC were treated with dose escalation from 36 Gy in 3 fractions to a total dose of 48 Gy in 3 fractions if dose-limiting toxicities were not suffered [105]. Patients were eligible for this trial if they had Child-Pugh score A or B , a solitary tumor less than 6 cm in size or three lesions with total diameter less than 6 cm, and adequate liver function. In this trial, patients were treated in the Elekta Stereotactic Body Frame with abdominal compression to minimize diaphragmatic motion to less than 0.5 cm. Patients had daily image guidance with cone-beam CT scans prior to the delivery of each fraction. The target volume was delineated based on CT-based imaging, with no clinical target volume expansion and a minimum of 5 mm axial and 10 mm craniocaudal planning target volume expansion. Patients with portal vein thrombosis were allowed on the protocol, and the entire length of the thrombus was treated with a 1 cm margin. Key normal tissue constraints were that 1/3 of the uninvolved liver received less than or equal to 10 Gy for Child-Pugh class A patients and that 1/3 of the uninvolved liver received less than or equal to 15 Gy for Child-Pugh class B patients. Renal constraints included less than 2/3 of the right kidney receiving greater than 15 Gy and 1/3 of the left kidney receiving greater than 15 Gy. The maximum bowel and stomach dose were 12 Gy. In this study, the dose was successfully escalated to patients with Child-Pugh class A to 48 Gy in 3 fractions without reaching dose-limiting toxicity. However, in patients with Child-Pugh class B cirrhosis, the maximum tolerated dose was 40 Gy in 5 fractions due to two patients suffering grade 3 liver toxicity. With long-term follow-up, the Indiana experience found positive rates of 2-year local control of 90 % among the treated population. There were no long-term grade 3 or higher non-hematologic toxicities, and 20 % of patients were found to experience progression in the Child-Pugh score at 3 months [106].
A second key Phase I/II trial was performed by Princess Margaret University and the University of Toronto [107]. In this trial, patients with Child-Pugh score A with no more than five liver tumors with a maximal dimension of 15 cm were enrolled. Patients in this trial were treated to a dose of 30–54 Gy in six fractions, with the maximum effective irradiated liver volume of 60 %. No patients in this trial suffered classic RILD or dose-limiting toxicity, with a decline in Child-Pugh score at 3 months occurring in 29 % of the cohort. Like the Indiana experience, the local tumor control was excellent at 87 % at 1 year. These two trials provide data for the efficacy for SBRT in the setting of well-controlled and designed clinical trials.
While these studies were limited to patients with preserved to mildly elevated liver function, there is evidence for the treatment of patients with Child-Pugh B7 or B8 with SBRT/SABR as well. The Princess Margaret group performed a prospective study with patients with Child-Pugh B7 or 8 with less than 10 cm of HCC tumor [108]. Patients received a median dose of 30 Gy in 5 fractions; however, as expected with their more fragile liver function, 63 % of the cohort had a decline in their Child-Pugh score at 3 months. Sorafenib is a tyrosine kinase inhibitor which is used in patients with advanced HCC, showing an improvement in overall survival compared to placebo. Currently an RTOG trial (RTOG 1112) is enrolling patients with advanced-stage HCC to daily sorafenib versus SBRT/SABR alone followed by daily sorafenib. The primary endpoint of the trial is overall survival with secondary endpoints evaluating the safety profile of SBRT/SABR plus sorafenib. This trial will potentially further expand the utilization of SBRT/SABR patients with advanced HCC.
SBRT/SABR for the Treatment of Liver Metastases
Because of its rich blood supply, hematogenous metastases to the liver are common among patients with solid organ malignancies [109]. Colorectal cancers are the most common primary malignancy to metastasize to the liver due to drainage via the portal circulation, with up to 50 % of patients suffering hepatic metastases within 5 years [110]. A subset of patients with metastatic disease present with oligometastases, a hypothesis popularized in 1995 by Hellman and Weichselbaum. It states that metastatic disease occurs in a stepwise manner, with limited metastases initially followed by progression to widespread disease [111]. Early in the spectrum, metastases may be limited in number and location [112]. Improvements in imaging including PET/CT and MRI have allowed for identification of isolated metastatic deposits with higher sensitivity and specificity than ever before. A significantly greater proportion of patients may be identified early in the metastatic spectrum and offered potentially curative local treatment with liver metastases.
Treatment of oligometastases was first performed via surgical metastasectomy with surgical resection of hepatic, pulmonary, or adrenal metastases having improved rates of survival with resection [113–115]. Furthermore, systemic therapy may convert patients with widely metastatic disease to a limited volume metastatic state, increasing the proportion of patients who may be candidates for early treatment of oligometastatic disease. Surgical metastasectomy is the standard of care in patients who are candidates; however, this is available only to approximately a quarter of patients with hepatic metastases due to the extent of disease or comorbidities [116]. RFA and TACE, much like utilized in hepatocellular carcinoma , are treatment options for patients with hepatic metastases as well.
Noninvasive treatment of hepatic metastases is also possible with external beam radiotherapy . Stereotactic body radiotherapy has allowed the delivery of high doses of therapy in single and multiple fractions with excellent rates of local control. A multi-institutional Phase I/II trial from the University of Colorado enrolled patients with 1–3 liver metastases from any solid tumor, cumulative maximum tumor diameter <6 cm, adequate liver and kidney function, and no chemotherapy 14 days before or after SBRT [47]. In the Phase I portion, the SBRT/SABR dose was escalated from 36 to 60 Gy in 3 fractions. Thirteen patients were treated with a dose of less than 60 Gy and 36 patients treated at 60 Gy, for a total of 63 hepatic lesions . Volume delineation was similar to that in the lung oligometastases trial, with the PTV defined as GTV expanded by 5 mm radially and 10 mm craniocaudally and 7 mm radially and 15 mm craniocaudally, with active breathing control and abdominal compression, respectively. At least 700 cc of normal liver had to receive a total dose <15 Gy, and the sum of the left and right kidney volume receiving 15 Gy had to be less than 35 %. With a median follow-up of 16 months, the 2-year actuarial in-field local control was 92 % with a median overall survival of 20.5 months. Treatment was well tolerated with one patient suffering grade 3 soft-tissue toxicity, no grade 4 or 5 toxicity, and no instances of radiation-induced liver dysfunction (RILD) .
Recently, interest has been increased in the delivery of single-fraction liver SBRT/SABR . Wulf et al. demonstrated that single-fraction doses of 26 Gy improved local control at 12 months to approximately 100 % with no grade 3 or higher toxicity [117]. More recently, SBRT/SABR was successfully escalated to 40 Gy in a single fraction with no grade 3 or higher toxicities related to treatment observed [118]. Furthermore, the 36-month rate of local control was 100 % showing an excellent opportunity to control liver metastases . Figure 8.2 shows dosimetry and beam geometry for single-fraction liver SBRT/SABR .


Fig. 8.2
Stereotactic body radiation therapy (SBRT) of a colorectal liver metastasis . (a) Beam arrangements for treatment of liver dome lesion. Diaphragmatic motion was limited by the use of a compression plate on the abdomen. (b) Dose distributions for treatment of large lesion in liver dome in axial, sagittal, and coronal planes , receiving 35 Gy in a single fraction
Specific Issues Associated with SBRT/SABR for Targets in the Liver
Liver SBRT/SABR for metastatic disease is often performed in patients without concomitant cirrhosis. Nonetheless, normal liver reserve, much like surgical resection , is a key consideration with treatment planning, with a minimal residual functional volume of approximately 700 cc desired. In patients with HCC, the doses delivered, as seen above, are lower than for metastatic disease due to the sensitive, cirrhotic liver.
Traditional SBRT/SABR is delivered via photon beams with energies between 6 and 18 MV. Patient immobilization is a key factor in the delivery of stereotactic treatment, with stereotactic frames with reference to the stereotactic coordinate system , a commonly utilized system. Motion management for treatment of the liver is essential, given the considerable motion of the organ and diaphragm. During CT simulation, the movement of the dome of the diaphragm should be visualized via fluoroscopy or alternative means with techniques to limit motion including breath-hold and abdominal compression. Target volume delineation of liver lesions is ideally done with registration of an abdominal MRI, done in the treatment planning position with motion management, if possible. Planning can be performed with noncoplanar 3D-conformal techniques, intensity-modulated radiation therapy, or volumetric-modulated arc therapy . Prescription isodose lines covering the PTV are between 60 and 90 %, and suggested dose constraints for adjacent normal structures for 1, 3, and 5 fractions are shown below in Table 8.1.
Table 8.1
Proposed dose constraints for SBRT /SABR treatments of 1, 3, and 5 fractions
Serial tissue | Volume (cc) | Volume max (Gy) | Max point dose (Gy)a | Endpoint (≥grade 3) |
|---|---|---|---|---|
One fraction | ||||
Spinal cord and medulla | <0.35 | 10 | 14 | Myelitis |
Esophagusb | <5 | 11.9 | 15.4 | Stenosis/fistula |
Heart/Pericardium | <15 | 16 | 22 | Pericarditis |
Rib | <5 | 28 | 33 | Pain or fracture |
Skin | <10 | 25.5 | 27.5 | Ulceration |
Stomach | <5 | 17.4 | 22 | Ulceration/fistula |
Bile duct | 30 | Stenosis | ||
Duodenumb | <5 | 11.2 | 17 | Ulceration |
<10 | 9 | |||
Jejunum/ileumb | <30 | 12.5 | 22 | Enteritis/obstruction |
Colonb | <20 | 18 | 29.2 | Colitis/fistula |
Parallel tissue | Critical volume (cc) | Critical volume dose max (Gy) | ||
Liver | 700 | 11 | Basic liver function | |
Renal cortex (right and left) | 200 | 9.5 | Basic renal function | |
Serial tissue | Volume (cc) | Volume max (Gy) | ||
Three fractions | ||||
Spinal cord and medulla | <0.35 | 15.9 | 22.5 | Myelitis |
Esophagusb | <5 | 17.7 | 25.2 | Stenosis/fistula |
Heart/pericardium | <15 | 24 | 30 | Pericarditis |
Rib | <5 | 40 | 50 | Pain or fracture |
Skin | <10 | 31 | 33 | Ulceration |
Stomach | <5 | 22.5 | 30 | Ulceration/fistula |
Bile duct | 36 | Stenosis | ||
Duodenumb | <5 <10 | 15.6 12.9 | 22.2 | Ulceration |
Jejunum/ileumb | <30 | 17.4 | 27 | Enteritis/obstruction |
Colonb | <20 | 24 | 34.5 | Colitis/fistula |
Parallel tissue | Critical volume (cc) | Critical volume dose max (Gy) | ||
Liver | 700 | 17.1 | Basic liver function | |
Renal cortex (right and left) | 200 | 15 | Basic renal function | |
Serial tissue | Volume (cc) | Volume max (Gy) | ||
Five fractions | ||||
Spinal cord and medulla | <0.35 | 22 | 28 | Myelitis |
Esophagusb | <5 | 19.5 | 35 | Stenosis/fistula |
Heart/pericardium | <15 | 32 | 38 | Pericarditis |
Rib | <5 | 45 | 57 | Pain or fracture |
Skin | <10 | 36.5 | 38.5 | Ulceration |
Stomach | <5 | 26.5 | 35 | Ulceration/fistula |
Bile duct | 41 | Stenosis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||