Institution
Number of cases
Median follow-up (months)
Fractionation scheme (cGy)
Total dose (cGy)
Percent local recurrence
Good/excellent cosmetic results
HDR brachytherapy
Ochsner Clinic [29]
26
75
400 × 8
3,200
2a
75a
National Institute of Budapest, Hungary Phase II Trial [30]
45
60
520 × 7
3,640
4.4
97
433 × 7
3,030
National Institute of Oncology, Budapest, Hungary Phase III [30]
221
30
520 × 7 (HDR)
3,640
0
NS
200 × 25b (EBRT)
5,000
<1
NS
London Regional Cancer Center London, Ontario [31]
39
20
372 × 10
3,720
2.6a
NS
79
52
400 × 8
3,200
1
100
Radiation Therapy Oncology Group [33]
66
78
340 × 10
3,400
3
–
MammoSite® (FDA approval trial) [34]
43
8
340 × 10
3,400
0
97
Tufts-New England Medical Center [35]
32
33
340 × 10
3,400
3
88
Medical College of Virginia/VCU [36]
44
42
0
80
LDR brachytherapy series
Ochsner Clinic [29]
26
75
>40 cGy/h
4,500
2a
75a
Guy’s Hospital [37]
27
72
40 cGy/h
5,500
37
83
120
82
52 cGy/h
4,992
1
91
Radiation Therapy Oncology Group [33]
33
85
–
4,500
12
NS
Massachusetts General Hospital [38]
48
23
50 cGy/h
5,000–6,000
0
92
External beam radiotherapy series
William Beaumont Hospital [39]
22
20
340–385 × 10
3,400–3,850
0
100
New York University [40]
47
18
600 × 5
3,000
0
All late toxicity ≤Grade 1
Intra–operative radiotherapy series
European Institute of Oncology, Milan Italy [41]
84
8
1,700–2,100 × 1
1,700–2,100
University College of London [42]
3
24
500–750 × 1
500–750
0
Most experience with APBI has been with interstitial brachytherapy, with most of that experience until recently being with the multi-catheter type (Table 16.1). The primary disadvantages of conventional multi-catheter brachytherapy are the complexity and invasiveness of the procedure. Conventional breast brachytherapy requires the use of up to 20 catheters or needles placed around the excision site (Fig. 16.1).
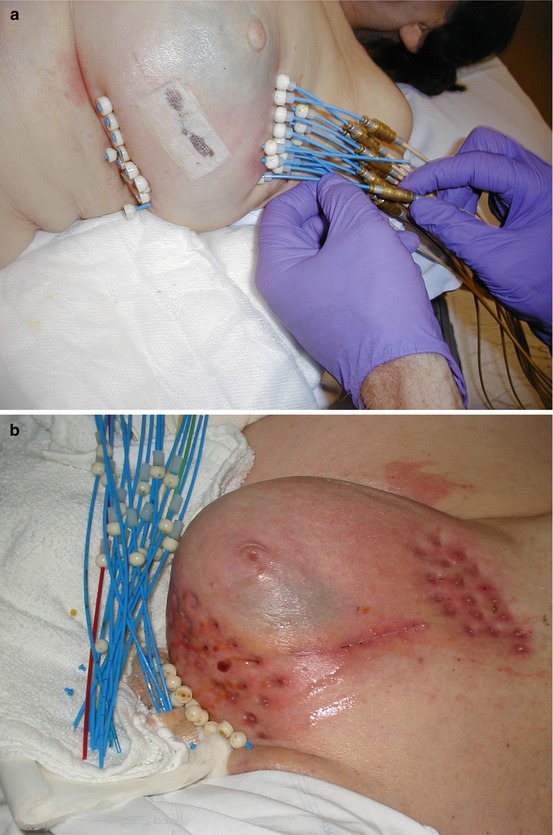

Fig. 16.1
Example of accelerated partial breast irradiation delivered using intersititial brachytherapy with multiple catheters. (a) The flexible hollow catheters are inserted around the lumpectomy cavity during breast conserving surgery. After CT-based treatment planning, these catheters are connected to a High-Dose Rate (HDR) remote after loading machine for delivery of the 10 treatments over 1 week. Immediately following completion of the tenth treatment the catheters are removed. (b) Shown is the trauma to the breast (and associated discomfort) along with the possible risk of infection and bleeding associated with interstitial brachytherapy APBI
There are several ways of placing these catheters, or needles, into the breast, and the procedure can be done either under local anesthesia with conscious sedation after surgery or under general anesthesia at the time of lumpectomy. These needles are inserted through the breast, and flexible catheters are threaded through the needles to cover the target area around the breast cavity. Interstitial brachytherapy has the advantages of high dose conformity to the target volume, independent of organ motion or respiration; delivery of additional simultaneous boost to the inner core of the target (the high risk area); and the ability to adapt to various patients’ anatomy and target shape (convexity). The interstitial brachytherapy experience provides the earliest clinical experience and has the longest follow-up clinical data for APBI. Nevertheless, this technique has not gained widespread popularity because of the relative complexity associated with performing an interstitial implant and the lack of significant patient interest in an additional invasive procedure with risk of infection, pain, and bleeding (Fig. 16.1b). As a consequence, multi-catheter APBI has been limited to only a handful of institutions.
The oldest brachytherapy APBI series comes from the Oschner Clinic [29] and the largest series from William Beaumont Hospital [11, 32]. At the Oschner Clinic, 51 women with 52 tumors were treated [29]. Eligibility criteria included intraductal or invasive carcinomas less than or equal to 4 cm in size, 0–3 positive axillary lymph nodes, and negative inked microscopic margins. Multiple plane interstitial implant was placed under direct visualization of the excision cavity or with ultra-sound guidance, and the catheters extended 2 cm beyond the cavity in all peripheral dimensions. Patients were assigned to low-dose-rate (LDR) and high-dose-rate (HDR) brachytherapy alternating in blocks of 10. LDR patients received 45 Gy in 3.5–6 days, while HDR patients received 32 Gy in 8 fractions over 4 days. At a median follow-up of 75 months, there were three grade 3 complications (5.8 % overall, 3.8 % LDR, 7.7 % HDR). Two HDR patients experienced severe fat necrosis, one requiring a mastectomy and the other a quandrantectomy with flap coverage. One LDR patient developed an abscess from an infected seroma at 4 months which was incised and drained. The rate of good/excellent cosmesis was 78 % in LDR and 67 % in HDR (p = 0.39). There has been only one local recurrence, located near the surgical scar occurring 78 months after radiotherapy.
In the William Beaumont Hospital Experience with multi-catheter brachytherapy APBI, 199 consecutive women with invasive early-stage breast cancer were treated from 1993 to 2001 [11, 32]. One hundred twenty patients were treated as in-patients with LDR, receiving 50 Gy over approximately 96 h. Seventy-nine were treated as out-patients with HDR, receiving either 32 Gy in 8 fractions of 4 Gy each or 34 Gy in 10 fractions of 3.4 Gy each, twice a day, with at least 6 h between fractions. One hundred fifty-eight patients met the strict eligibility criteria which included infiltrating ductal carcinomas <3 cm, surgical margins clear by at least 2 mm, age >40, no extensive intraductal component (EIC-), and no clinically significant lobular carcinoma in situ. Initially patients with 1–3 involved axillary lymph nodes were allowed. The protocol was modified after the first 50 patients, and the subsequent 149 patients were required to be node negative. At a median follow-up of 65 months (range, 12–115 months), a total of five ipsilateral recurrences were observed; two were located near the original primary site and three were located elsewhere in the breast. The mean time to local failure was 5 years (range, 1.5–7.6 years).
In recent years, the development of the MammoSite® (Proxima Therapeutics, Inc., Alpharetta, GA) balloon brachytherapy technique has increased interest in APBI.
This brachytherapy applicator was developed as a more “user friendly” technique in which a single balloon is placed in the excision cavity [34]. While nursing care for the single catheter is less and patients may be more comfortable there remain several drawbacks. This technique requires a second surgical procedure to place the catheter and wound care for 1–2 weeks. Some patients are candidates for APBI, but they are not candidates for Mammosite due to geometric factors such as small breast size, too much air/gas in the cavity, or the cavity is too close to the chest wall or skin. The applicator is not suitable for lesions close to the skin surface or for irregularly shaped cavities to which the balloon does not conform. The Mammosite has to be inflated for the entire 7–14 day treatment duration, which can be uncomfortable, and the catheter entry point can serve as a source of infection. Typically, patients with an inserted device are placed on prophylactic antibiotics. All being said, a recent publication from the American Society of Breast Surgeons Mammosite Breast Brachytherapy Registry Trial reported a 91 % good to excellent cosmetic result at a mean follow-up of 54 months (range, 0–86 months) in a treated population of 1,449 women with early breast cancer [43].
Three-dimensional (3D) conformal external beam radiation therapy has also been pursued as a technique to treat patients with APBI using a similar, shortened treatment schedule. This 3D technology is readily available in the majority of radiation facilities allowing many more radiation oncologist groups that do not perform brachytherapy to deliver APBI. Perhaps the greatest advantage of this method of APBI is the fact that no additional invasive procedure is required. However, conventional radiation equipment cannot pinpoint radiation delivery as accurately as brachytherapy. Therefore, a large margin is required to account for the set-up uncertainty and for respiratory motion during treatment. This margin leads to a larger treatment volume and more irradiation of the normal structures (lung, chest wall, skin, heart). Indeed, there are now published concerns of toxicities with unacceptable cosmesis seen in populations of women who elected for APBI using 3-D conformal external beam approach [44, 45].
The NSABP B39/RTOG 0413 trial randomizes select patients with stage 0, I and II breast cancer after lumpectomy to either WBI or APBI with the goal of documenting the long-term equivalence of APBI to WBI. Patients randomized to the APBI arm are treated with either interstitial brachytherapy with catheters/needles; intracavitary brachytherapy with the Mammosite balloon catheter; or 3D conformal external beam radiation therapy. A recent publication by Patel et al. [46] reported the 5-year follow-up for 273 patients treated with brachytherapy using either multicatheter interstitial brachytherapy (n = 247) or Mammosite (n = 26). The patients received 32–34 Gy in 8–10 twice daily fractions using high-dose-rate 192Ir brachytherapy. All patients met the initial inclusion criteria for the trial and were separated into either a high- or low-risk group. The high-risk patients (n = 90), who represent the cohort that remained eligible for the intergroup trial, satisfied one or more of the “high-risk” criteria: age <50, estrogen receptor negative, and/or positive lymph nodes. The low-risk patients comprised the remainder of the cohort (n = 183). At a median 48.5 months follow-up for the entire cohort, no significant difference was found in outcomes at 5 years between the low- and high-risk groups with a local control rate of 97.8 % vs 93.6 %, crude local recurrence rate of 2.2 % (n = 4) vs 4.4 % (n = 4), and overall survival rate of 92.1 % vs 89.5 %, respectively.
A Phase II Electron Intraoperative radiotherapy (ELIOT) trial is underway in Europe. Patients over 55 years of age with tumors ≤2.5 cm are randomized to WBI (50 + 10 Gy boost) versus 21 Gy single fraction electron intra-operative radiotherapy (IORT). The accrual goal for the study is 800 patients. To perform IORT, a mobile linear accelerator with a robotic arm is used to deliver a single-fraction electron dose to the involved quadrant of the breast after quadrantectomy. An aluminum/lead disc is placed between the breast and pectoralis muscle to shield the chest wall and lungs. Initial experience was as an “up-front” boost to anticipate WBI with dose escalation from 10 to 15 Gy. This was well tolerated, and the approach changed to sole treatment with APBI using a single fraction of IORT. Dose was escalated from 17 to 21 Gy without unexpected acute toxicity. It is estimated that 21 Gy in a single fraction is radiobiologically equivalent to 60 Gy in 30 fractions of 2 Gy/fraction over 6 weeks [41].
The advantages of this technique are convenience to patient, minimal risk of radiation to surrounding normal structures (lung, heart and breast), lower cost, and no delay to adjuvant chemotherapy when needed. The critics of this technique cite potential under-coverage of the target volume (due to unknown final margin status and the directional nature and energy of the electron beam), additional use of operating time and patient time under general anesthesia, as well as cost of purchasing the IORT machine.
16.4 Stereotactic Body Radiotherapy Delivery Of APBI
Stereotactic body radiotherapy (SBRT) offers the potential of combining the benefits of the precisely targeted dose delivery of an interstitial brachytherapy APBI with the non-invasiveness of external beam radiation therapy. SBRT delivers a highly conformal dose that mimics the dosimetry of a breast interstitial brachytherapy implant. In order to accomplish this SBRT must employ image-guided delivery, typically via tracking of fiducial markers which in the case of APBI are implanted during tumor resection keeping the subsequent SBRT non-invasive. The CyberKnife (Accuray Incorporated, Sunnyvale, CA) is a frameless robotic stereotactic radiosurgery system that provides image-guidance for continuous tracking of target motion with respiration and patient movement [47]. Recently, the CyberKnife has been explored for SBRT delivery of APBI due to its image-guidance capability which allows continuous tracking of the target’s motion with respiration and patient movement. This allows the margins to be reduced to a minimum, thus sparing surrounding critical structures from undue radiation exposure. Researchers at the University of Texas Southwestern Medical recently compared CyberKnife SBRT APBI and 3D-CRT treatment plans. They found that the SBRT APBI treatment plans achieved highly conformal target coverage and reduced the dose to nearby organs at risk relative to 3D-CRT plans [14]. Similarly, a treatment planning comparison from Fox Chase Cancer Institute concluded that the CyberKnife’s more conformal dose could result in reduced toxicity by a reduction in dose to surrounding breast tissue [13]. Figure 16.2 presents an SBRT APBI treatment plan in comparison with conventional radiation treatment plans highlighting the dose conformality of the SBRT APBI treatment plan.
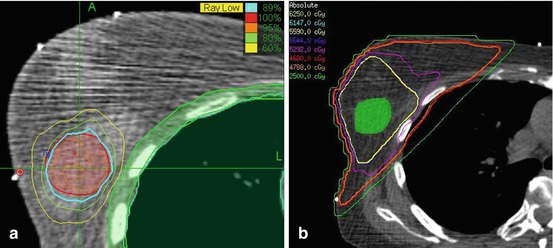

Fig. 16.2
Comparison of (a) an SBRT APBI treatment plan to that of (b) a conventional radiation treatment plan. Note the high conformality of the SBRT APBI treatment plan’s isodoses
At our institution, we have treated 21 patients with CyberKnife delivered SBRT. As experience and knowledge of SBRT APBI delivery grows, this treatment paradigm will evolve. The following presents a guide to how we currently perform SBRT APBI. While our experience has focused on the use of the CyberKnife for delivery of SBRT APBI we have attempted, as much as possible, to ensure this guide is informative for delivery of SBRT APBI with other devices. However, it is imperative that caution be employed to ensure that suitable motion tracking occurs to ensure the accuracy of dose delivery to the target while sparing nearby critical structures.
16.5 SBRT APBI Patient Eligibility
Our patient selection criterion incorporates patients considered “suitable” or “cautionary” candidates as outlined in the ASTRO consensus statement for APBI [48].
greater than 45 years of age
stage T1, T2 or Tis without metastases
histologically confirmed invasive non-lobular carcinoma or ductal carcinoma in situ (DCIS) of the breast
lesion size must be less than 3 cm and treated with wide excision.
patients with invasive tumors undergo an axillary sentinel node procedure or axillary dissection.
negative (>2 mm) microscopically assessed surgical margins for both invasive carcinoma and DCIS with no known unresected residual carcinoma or diffuse suspicious microcalcifications. If there is an extensive intraductal component (EIC +), the total size of the EIC and primary invasive tumor should be less than 3 cm, and the post-operative mammogram must show no evidence of suspicious residual abnormality. If the cancer presented with malignancy-associated microcalcifications then they must have a negative post-operative mammogram or specimen radiograph demonstrating removal of all microcalcifications.
16.6 Exclusion Criteria
pregnancy
collagen vascular disease
prosthetic augmentation implants
prior radiation therapy to the treated breast
invasive lobular or multicentric carcinoma
histologically confirmed positive axillary lymph nodes
tumors that involve the skin, that invade the chest wall/muscle, or that have diffuse suspicious microcalcifications on mammography
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree