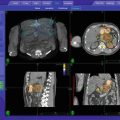
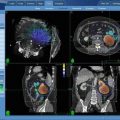
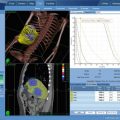
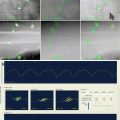
Fig. 17.1
Representative treatment plan of recurrent soft tisuue sarcoma SBRT reirradiation using the CyberknifeTM technique
17.7 Toxicity
Patients will need to be monitored during the treatment course with documentation of acute toxicity. No treatment related toxicities are usually seen other than mild fatigue. Brisk skin reaction can be anticipated with extremity superficial sarcomas. Fibrosis and lymphedema are long term concerns. Nausea has been reported in abdominal sarcomas and is managed conservatively with prophylactic or therapeutic antiemetics.
17.8 Post Treatment Care
Periodic clinical and radiological follow up is warranted based on institutional protocol. A 1 month baseline toxicity and response assessment clinical follow up and MRI, followed by 3–6 month assessment in the first couple of years and less often thereafter is advised. Adverse events and radiological assessments need to be carefully monitored and recorded.
17.9 Future Directions
The role of SBRT for sarcomas is evolving. Systematic study on prospective studies are warranted to assess comparative effectiveness, toxicity, cost benefit and quality of life benefits, Better understanding of dose fractionation and radiobiology will help define definitive role for SBRT for sarcomas.
References
1.
2.
Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203.PubMed
3.
Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14(3):859–68.PubMed
4.
5.
6.
7.
8.
9.
10.
Potluri S, Jefferies SJ, Jena R, Harris F, Burton KE, Prevost AT, et al. Residual postoperative tumour volume predicts outcome after high-dose radiotherapy for chordoma and chondrosarcoma of the skull base and spine. Clin Oncol (R Coll Radiol). 2011;23(3):199–208.CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree