Organ
Dose
Dose (EQD2)
Author(s)
Brachial Plexus
Dmax
60 Gy
Emami B, Lyman J, Brown A, et al. (1991)
Dmax
66 Gy
Truong MT, Nadgir RN, Hirsch AE, et al. (2010)
Brain
<55/52
Hasselt (1999), Serre (2007)
Dmax
45 Gy
Emami B, Lyman J, Brown A, et al. (1991)
Dmax
55 Gy
Armstrong CL, Hunter JV, Ledakis GE, et al. (2002)
Dmax
72 Gy
Lawrence YR, Li XA, el Naqa I, et al. (2010)
Brain Stem
54
Kam (2003)
Dmax
54 Gy
Emami B, Lyman J, Brown A, et al. (1991)
V60
<1 ml
Debus J, Hug EB, Liebsch NJ, et al. (1999)
Chiasm
54/55
Kam (2003), Serre (2007)
Cochlea
45/55
Bhide (2006), Bhandare (2000)
Dmean
40 Gy
Fleury B, Lapeyre M (2010)
Dmean
45 Gy
Bhandare N, Jackson A, Eisbruch A. et al. (2010)
Constrictor M
Dmean
55 Gy
Levendag PC, Teguh DN, Voet P, et al. (2007) [14]
NTCP 25
56 Gy
Eisbruch A, Kim HM, Feng FY, et al. (2011)
NTCP 50
63 Gy
Eisbruch A, Kim HM, Feng FY, et al. (2011)
Cornea
Dmax
40 Gy
Marchand V, Dendale R (2010)
Frontal lobe
<60
Serre (2007)
Pituitary
<55
Serre (2007)
Lacrimal gland
30
Durkin (2007), Weber (2006)
Dmax
35 Gy
Durkin SR, Roos D, Higgs B, et al. (2007)
Dmax
40 Gy
Parsons JT, Bova FJ, Mendenhall WM, et al. (1996)
Larynx
Dmax
64 Gy
Debelleix C, Pointreau Y, Lafond C, et al. (2010)
Dmean
40 Gy
Debelleix C, Pointreau Y, Lafond C, et al. (2010)
Lens
8–10/12
Serre (2007), Hein (2005)
Dmax
10 Gy
Marchand V, Dendale R (2010)
Mandibular bone
Dmax
60–70 Gy
Sargos P, Mamou N, Dejean C, et al. (2010)
Mucosa
65–77
Small (2006)
Muscle
70
Small (2006)
Optic Chiasma
Dmax
50 Gy
Emami B, Lyman J, Brown A, et al. (1991)
Dmax
52 Gy
Hoppe BS, Nelson CJ, Gomez DR, et al. (2008)
Optic nerve
54
Hoppe (2008)
Oral cavity
Dmean
50 Gy
Little M, Schipper M, Feng FY et al. (2011)
Parietal lobe
<60
Serre (2007)
Parotid
26
Eisbruch (2003)
Dmean
26 Gy
Murdoch-Kinch CA, Kim HM, Vineberg KA, et al. (2008)
NTCP 17–26
25–30 Gy
Dijkema T, Raaijmakers CP, Ten Haken RK, et al. (2010)
NTCP 50
39.9 Gy
Dijkema T, Raaijmakers CP, Ten Haken RK, et al. (2010)
Peripheral nerves
65–77
Small (2006)
Dmax
60 Gy
Henriques de Figueiredo B, Huchet A, et al. (2010)
Pituitary gland
Dmax
30–40 Gy
Bhandare N, Kennedy L, Malyapa RS, et al. (2008)
Retina
50/55
Monroe 2005, Serre (2007)
Dmax
45 Gy
Emami B, Lyman J, Brown A, et al. (1991)
Dmax
50 Gy
Monroe AT, Bhandare N, Morris CG et al. (2005)
Spinal cord
50
Hasselt (1999)
Dmax
50 Gy
R.B. Marcus, R.R. Million (1990)
Submandibular gland
Dmean
39
Murdoch (2008)
Temporal lobe
<60
Kam (2003)
Temporomandibular joints
60
Serre (2007)
Dmax
60 Gy
Emami B, Lyman J, Brown A, et al. (1991)
14.6 Erasmus MC Brachytherapy Experience—Basis for SBRT Boost
Patients series from 1991 to 2007 treated with brachytherapy (high tumor doses in relatively small volumes) were compared with the non-brachytherapy treated patients. The standard radiotherapy schedule for oropharyngeal cancer (OPC) at the Erasmus MC–Daniel den Hoed Cancer Center consists of 46 Gy of intensity-modulated radiotherapy (IMRT) with or without concomitant chemotherapy when indicated, followed by a pulsed-dose-rate or high-dose-rate brachytherapy boost (BTB; 20–22 Gy). Between 1991 and 2005, a total of 336 patients with primary OPC were treated with this protocol at the Erasmus MC–Daniel den Hoed Cancer Center, with excellent results. The actuarial 5-year LC, DFS and OS rates were significantly better for the BT boost, compared with the non-BT boost (LC 84 % vs. 60 %, p < 0.001, DFS 59 % vs. 43 %, p = 0.0004, and OS 64 % vs. 39 %, p < 0.001) [14].
Responses were are also assessed with validated QoL questionnaires (Table 14.2). In short, best QoL was observed for patients treated with maximal conformality, in sequential treatments using boost techniques such as brachytherapy, and stereotactic radiation. Chart review revealed that roughly 31 % of patients experienced moderate to severe dysphagia (RTOG grade 3 and 4). When grouped by the boost technique BT vs. non-BT, severe dysphagia (problem score of QoL H&N35 with swallowing item ≥50) was observed in patients with TF and/or SP tumors in 19 % and for BOT tumors in 22 %. For the non-BT group, severe dysphagia was found in 30 % of the TF/SP tumors and in 47 % for the BOT tumors. A further breakdown of the non-BT group with respect to the booster technique used, showed severe dysphagia in 42 % for P-O, 25 % for 3DCRT, and 25 % for IMRT. Because of the reported dose-effect relationships in HNC, the balance between local control and late side-effects deserves specific attention. We published before a dose-effect relationships for normal tissues for swallowing problems (Fig. 14.1) [14, 31].
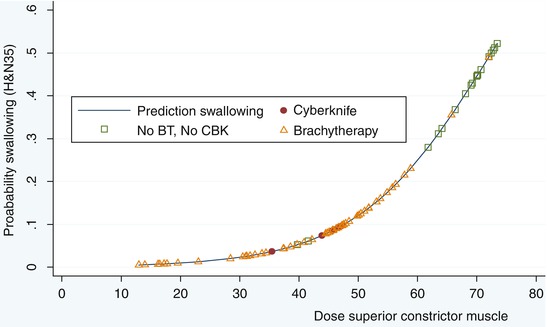
Table 14.2
Mean quality of life scores for patient with cancers in the oropharynx treated by RT, using different RT boost techniques
Boost | First series (46/2 Gy) | QLQ-C30 global health statusa | H&N35 swallowingb | H&N35 sticky salivab | H&N35 dry mouthb | H&N35 painb |
|---|---|---|---|---|---|---|
IMRT/3DCRT | IMRT/3CRT | 72 | 32 | 59 | 67 | 27 |
Par-Opp | 33 | 50 | 100 | 100 | 25 | |
BT | IMRT/3DCRT | 74 | 13 | 25 | 38 | 13 |
Par-Opp | 74 | 26 | 46 | 61 | 18 | |
SRT(+CBK) | IMRT/3DCRT | 71 | 15 | 47 | 58 | 26 |
Par-Opp | Par-Opp | 59 | 46 | 71 | 77 | 33 |

Fig. 14.1
Significant dose-effect relationship for the dysphagia complaint category EORTC H&N35 item and dose in superior constrictor muscle. Patients can be subdivided in those treated by a brachytherapy boost and those treated by 3DCRT boost or by IMRT boost. BT brachytherapy, CBK cyberknife
14.7 Toxicity Outcomes
Swallowing is a complex action requiring coordination between sensory input and motor function of the swallowing apparatus [32]. Intensification of therapy for head and neck cancer in general, either by altered fractionation RT schemes and/or by the addition of concomitant chemotherapy, results in improved locoregional tumor control [33–35], and increase of late sequelae, such as dysphagia. In general, the prevalence of dysphagia is probably being underreported because of its (sometimes) clinically silent nature, but can be as high as 50 % [35–38].
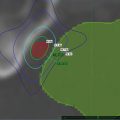
Levendag and colleagues explored the relationships between the mean total dose received by the five swallowing muscles to the responses of the three—dysphagia related—QoL questionnaires (mean QoL scores; H&N35, PSS, & MDADI), per tumor site (i.e. the TF and/or SP or BOT), and per treatment technique (BT vs. non-BT) [14]. One hundred and fifty five of the 336 patients fulfilled the criteria and were disease free with a minimum follow-up of 1 year. Mean Primary treatment sites were Tonsillar Fossa and/or Soft Palate (TF/SP) (n = 108), or Base of Tongue (BOT) (n = 47). 119/155 (77 %) of patients were BT, 48 boosted by non-BT techniques, boosted stage III&IV.107 patients. More complaints were reported with higher doses, in particular with regard to the superior- and medial constrictor muscles. Figure 14.4 shows an example of the dose-effect relationship computed by logistic regression. The steepness of the curve from 60 Gy, can be expressed by 20 % increase per 10 Gy. We speculate this increase in dysphagia (high dose, no BT, bilateral neck irradiation, no ND) has to do with the increase in irradiated volume and radiation dose. Xerostomia and dysphagia are also strongly correlated [31]. The treatment regime EBRT (or currently IMRT) combined with a BT boost has been applied in our institute preferentially over a great number of years for a variety of reasons: with regard to tumor control, HDR/PDR fractionation is given in an accelerated fashion with intrinsic dose escalation. A high degree of conformality is obtained (accurate CTV delineation, no PTV margin) with rapid dose fall-off. When grouped by boost technique (Table 14.2), severe dysphagia was observed less when BT was used as a boost technique. It is conceivable that such toxicity benefits from more conformal therapy could be achieved similarly with SBRT.
14.8 Erasmus MC SBRT Experience
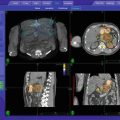
Given the limitations of the organ preservation protocol (adverse late side effects and costs), improvements of the therapeutic ratio by changing (parts of) the techniques and/or modalities was anticipated. In 2005, an SBRT system (Cyberknife) was installed at our institution In the revised organ preservation protocol (Fig. 14.2), the following basic premises were taken into consideration:
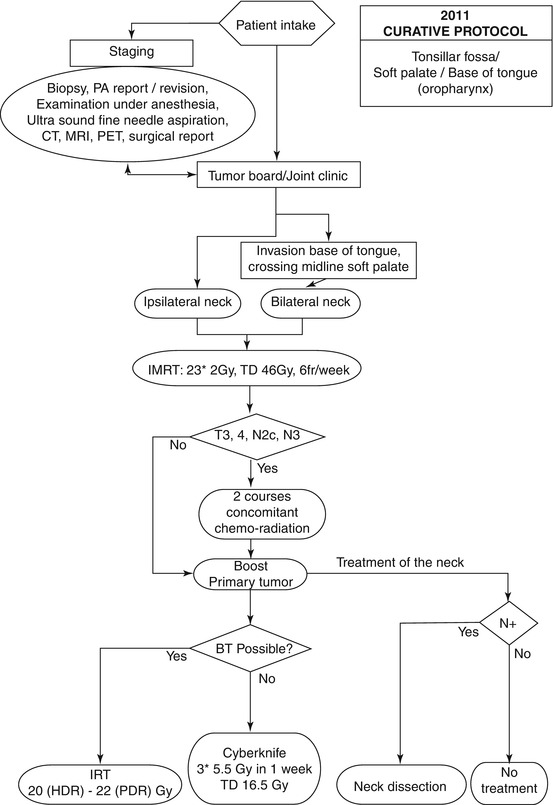

Fig. 14.2
Flow chart of oropharyngeal cancer treatment at the Erasmus MC–Daniel den Hoed Cancer Center. BT brachytherapy, CT computed tomography, HDR high-dose rate, IMRT intensity-modulated radiotherapy, IRT interstitial radiation therapy, MRI magnetic resonance imaging, PET positron emission tomography, PDR pulsed-dose rate, TD total dose, PA pathology
Since only 6 recurrences (4 %) were observed in the 149 electively treated CL necks, and no relapses were seen in the 29 non-treated CL necks, we suggest not to treat the contralateral neck, unless either the tumor extends beyond the midline of the soft palate (uvula) and/or invades a significant part of the ipsilateral base of tongue
With the currently available CT-based neck level definitions, more conformal contours, that is tighter boundaries, around the CTV can be designed [24]. This way critical structures can be avoided more easily.
Because of a number of conditions, some patients are non-eligible for BT, e.g.:
Patients with extensive, bulky tumors at the time of the implant
Tumors encroaching upon the ascending ramus of the mandible
Difficult to implant cavities (tonsillar fossae) after a recently performed tonsillectomy
Tumors with parapharyngeal extension
Patients non-eligible for surgery [i.e. anaesthesia] per se,
Patients refusing surgery.
Some of the patients not eligible for brachytherapy or surgery can be dealt with in principle by using the Cyberknife.
14.9 Overview of SBRT for Primary Head and Neck Cancers
Kodani and colleagues [39] evaluated the efficacy and safety of stereotactic body radiation therapy for patients with head and neck tumors. Included were 34 patients treated with Cyberknife of which 21 of them had prior RT. Treatment sites were orbit [7], cervical lymph nodes [6], nasopharynx [5], oropharynx [4] and others [12]. The prescribed dose ranged from 20 to 42 Gy (median, 30 Gy) in 3–8 fractions for consecutive days. The target volume ranged from 1 to 78 cm [3] (median, 11.6 cm [3]) and the median follow-up was 16 months. The authors reported that treatment was well tolerated without significant acute complications in any cases. Complete response rate and partial response rate were 32 and 39 %, respectively. The overall survival (OS) rates were 71 and 58 % at 12 and 24 months, respectively. The OS was better in patients without prior radiotherapy within the previous 24 months or in case of smaller target volume. Six patients suffered severe late complications which all had prior radiotherapy, and two of them developed massive hemorrhage in the pharynx and both died of this complication five and 28 months, respectively, after SBRT. The authors suggest that SBRT is an effective treatment modality for head and neck tumors, however, re-irradiation has significant risk of severe and even fatal late complications in the form of necrosis and hemorrhage in re-irradiated areas. Hara and colleagues [40] determined long-term outcomes in 82 patients receiving SBRT as a boost following external beam radiotherapy for locally advanced nasopharyngeal carcinoma between September 1992 and July 2006. Nine patients had T1, 30 had T2, 12 had T3, and 31 had T4 tumors. Patients received 66 Gy of EBRT followed by a single-fraction SBRT boost of 7–15 Gy, delivered 2–6 weeks after EBRT. Seventy patients also received cisplatin-based CHT delivered concurrently with and adjuvant to RT. Only 1 local failure in a patient with a T4 tumor at a median follow-up of 40 months was described. At 5 years, the freedom from local relapse rate was 98 %, freedom from nodal relapse 83 %, freedom from distant metastasis 68 %, freedom from any relapse 67 %, and overall survival 69 %. Late toxicity included radiation-related retinopathy [3], carotid aneurysm [1], and radiographic temporal lobe necrosis (10 patients), of whom 2 patients were symptomatic with seizures. Of 10 patients with temporal lobe necrosis, 9 had T4 tumors. The authors concluded that SBRT boost after EBRT provides excellent local control for patients with nasopharyngeal cancer although better systemic therapies for distant control are needed.
Kawaguchi and colleagues [41] published on the effect of SRT on local control and organ preservation in cases of primary squamous cell head and neck cancer. In this retrospective study, 14 patients with a mean age of 73 years were treated between March 2006 and September 2007 with SRT. The staging consisted of T2 [5], T3 [3], T4 [6], N0 [13], and N1 [1]. Marginal doses were 35–42 Gy in 3 or 5 fractions. Significant tumor reduction was noted at the third month of follow-up with 5 complete responses and 9 partial responses. At a mean follow-up of 36 months the LC and OS rates were 71 % (10/14) and 79 % (11/14), respectively. The authors concluded that it is feasible to use SBRT for primary HNC and its potential benefit in LC and organ preservation.
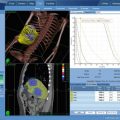
Al-Mamgani and colleagues prospectively assessed the outcome and toxicity of frameless stereotactic radiotherapy (SRT) as a treatment option for boosting primary oropharyngeal cancers in 51 patients (stage T1–T4N0–N + oropharynx) who were not suitable for brachytherapy boost [42]. In 29 patients technically not suitable for implantation (56 %), 9 patients (18 %) were medically unfit to undergo the procedure of BT because of major comorbidity, in 4 patients (8 %) BT was done because of logistical problems, and in 9 patients (18 %) because of a combination of the above-mentioned factors. They received SBRT boosts (3 fractions of 5.5 Gy), prescribed to the 80 % isodose-line enclosing 100 % of the CTV and at least 95 % of the PTV, after an accelerated scheme of 46 Gy IMRT to the primary tumor and neck (when indicated) in 2 Gy fractions daily. The planning treatment volume (PTV) included a margin of 3 mm beyond the CTV to account for different targeting uncertainties. The boost to the primary tumor consisted of 3 fractions given within 1 week on each consecutive day, for instance, directly after completion of the first part of the treatment with 46 Gy of IMRT within a median overall treatment time of 32 days. Treatment duration varied between 45 and 60 min, depending on the number of beams used and the patient’s compliance in finishing the treatment without interruption. The RT was combined with concomitant chemotherapy in patients with T3/T4 and/or 2c/N3 tumors (Fig. 14.2). The median age of the investigated group was 60 years with oropharyngeal cancer situated in the tonsillar fossa in 49 %, with T2 tumors in 53 %, and node-negative in 65 % of the patients. After a median follow-up of 18 months, the 2-year actuarial rates of LC, DFS, and OS were 86, 80, and 82 %, respectively, and the 3-year rates were 70, 66, and 54 %, respectively (Fig. 14.3). The overall 2-year cumulative incidence of grade ≥2 late toxicity was 28 %. Of the patients with 2 years with no evidence of disease (n = 20), only 1 patient was still feeding tube dependent and 2 patients had grade 3 xerostomia. Complete response was achieved in 49 patients (96 %) and partial response in 2 patients (4 %). Both patients with partial response had T3 and T4 disease, but could not receive chemotherapy in combination with radiotherapy because of comorbidities, and have developed a local recurrence after 5 and 6 months. Among all patients, 7 events (5 local recurrences, 1 regional recurrence, and 1 distant metastasis) were reported. Two patients were salvaged successfully with surgery (one local and one regional recurrence) and, at the time of writing, are still alive with no evidence of disease progression. Four patients with local recurrences and the patient with distant metastasis eventually died of their disease. Three other patients died of intercurrent disease or second malignancy without any evidence of relapse. Patients with T1/T2 disease had better LC than patients with T3/T4 (1 vs. 4 local recurrences, respectively; p = 0.08). The most serious acute toxicities were grade 3 dysphagia (feeding tube dependent) in 45 %, grade 3 mucosal toxicity (confluent mucositis) in 25 %, and grade 3 skin toxicity (moist desquamation) in 16 % of the patients (Table 14.3). Seven patients (14 %) required hospitalization (5 days (range, 2–16 days)) during treatment because of severe mucositis, dysphagia, and weight loss [5], neutropenic fever [1], or intercurrent infection [1]. Tumor stage, tumor involving the base of tongue, the use of chemotherapy, and bilateral neck irradiation were significant predictors for the need of tube feeding at univariate analysis (Table 14.4). At multivariate regression analysis, only chemotherapy and bilateral neck irradiation remain significant within the multivariate model; the corresponding odds ratios were 12.5 and 9.6, respectively. No grade 4 or 5 late toxicity was seen in the 50 patients with a minimum event-free follow-up of 6 months and in 32 patients with a minimum event-free follow-up of 12 months. The 2-year cumulative incidence of grade ≥2 late toxicity was 28 %. At 6 months, 2 patients reported grade 3 xerostomia and dysphagia; 1 of these patients had the feeding tube in place 2 years post treatment (Table 14.5). The 2-year cumulative incidence of grade ≥2 dysphagia and xerostomia was 15 and 28 %, respectively. No cases of trismus, bone, or soft-tissue necrosis were reported. Al-Mamgani and colleagues concluded that patients with oropharyngeal cancer who are not suitable for BT boost could safely, and effectively receive boosts by SBRT. Given the high fraction size (5.5 Gy) and the shorter overall treatment time in patients treated with SBRT (median overall treatment time, 32 days), compared with those treated with an IMRT boost at Erasmus MC with an accelerated schedule of 6 fractions per week (median overall treatment time, 42 days), the total biologically equivalent doses in 2 Gy/fx of the schedule used in the study (46 Gy of IMRT followed by 16.5 Gy with SBRT) would be 73 Gy. The treatment outcomes are fairly comparable to those in oropharyngeal cancer patients who received boosts by BT at our institution (87, 74, and 80 %, respectively) but compare favorably to those in oropharyngeal cancer patients treated with IMRT or 3DCRT boost (64, 52, and 60 %) [43, 44]. Teguh and colleagues reported grade 3 and 4 dysphagia from chart review in 24/132 (18 % of the patients treated with IMRT or 3DCRT [19], compared to the 2-year cumulative incidence of grade ≥2 dysphagia and xerostomia of 15 and 28 %, respectively in Al-Mamgani’s study. The dose conformality afforded by the use of SBRT and reduced CTV)/PTV margins (from 5 mm for IMRT planning to 3 mm for Cyberknife planning) do seem to have a substantial effect on the dose received by the swallowing muscles and parotid glands, which could explain the reduced late toxicity in patients treated with SBRT, as opposed to those treated with an IMRT or 3DCRT boost [19]. In patients treated with SBRT, the mean dose to the swallowing muscles was reduced on average by 20–25 % and the mean dose to the contralateral parotid gland by 15 %. The lower incidence of dysphagia in Al-Mamgani’s group of patients might also be partly attributed to the reduced incidence of xerostomia in patients who received boosts with SBRT, as dysphagia-related complaints have been shown to increase significantly in patients with reduced production of saliva after chemo (radiation) [19, 22]. Uno and colleagues published a retrospective study of initial results of a Cyberknife boost for tumors in the head and neck area with ten patients [45]. A variety from 9 to 16 Gy in 3–4 fractions was given. They found in three patients local progression when 50 Gy + 15 Gy (4 fraction), 50 Gy + 14 Gy (4 fraction), and 40 Gy + 16 Gy (4 fraction) was used. All progressions were within the CTV of SBRT boost. Dose escalation and/or change in the fractionation schedule in the SBRT boost component is proposed. They reported no grade 3 or worse toxicity directly attributable to the SBRT boost.

Fig. 14.3
Treatment schedule SRT + HT
Table 14.3
Acute radiation toxicity Erasmus SBRT Boost experience, scored according to Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) (N = 51)
Acute side effects | No. of patients (%) |
|---|---|
Dermatitis | |
Grade 2 | 30 (59 %) |
Grade 3 | 8 (16 %) |
Mucositis | |
Grade 2 | 31 (61 %) |
Grade 3 | 13 (25 %) |
Salivary gland changes | |
Grade 2 | 10 (19 %) |
Grade 3 | 0 |
Dysphagia | |
Grade 2 | 20 (39 %) |
Grade 3 | 23 (45 %) |
Pain | |
Grade 2 | 16 (31 %) |
Grade 3 | 0 |
Taste alteration | |
Grade 2 | 15 (29 %) |
Grade 3 | 0 |
Nausea | |
Grade 2 | 5 (10 %) |
Grade 3 | 0 |
Table 14.4
Results of logistic regression analysis for the correlation between different patients’ characteristics and the incidence of acute grade 3 dysphagia (feeding tube dependent)—erasmus SBRT boost experience
Patient characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
OR | p | OR | p | |
Age | 1.09 | 0.87 | ||
Sex | 2 | 0.28 | ||
Tumor stage | 3.9 | 0.02 | 0.7 | |
Involvement of BOT | 3.2 | 0.05 | 0.9 | |
Chemotherapy | 20.7 | 0.006 | 12.5 | 0.02 |
Bilateral neck irradiation | 16.5 | 0.01 | 9.6 | 0.04 |
Table 14.5
Late radiation toxicity, scored according to common terminology criteria for adverse events version 3.0 (CTCAE), in patients with a minimum event-free follow-up of 6 and 12 months—erasmus SBRT boost experience
Late side effects | 6 months (n = 50) | 12 months (n = 32) |
|---|---|---|
No. of patients (%) | No. of patients (%) | |
Dysphagia | ||
Grade 2 | 7 (14 %) | 4 (12 %) |
Grade 3
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||