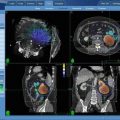
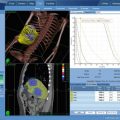
Fig. 10.1
Adaptive tolerance based stereotactic body radiotherapy dose prescription, showing a graphical depiction of the relationship between the duodenum and pancreatic tumor (red) that is used to determine each of the three prescribed doses
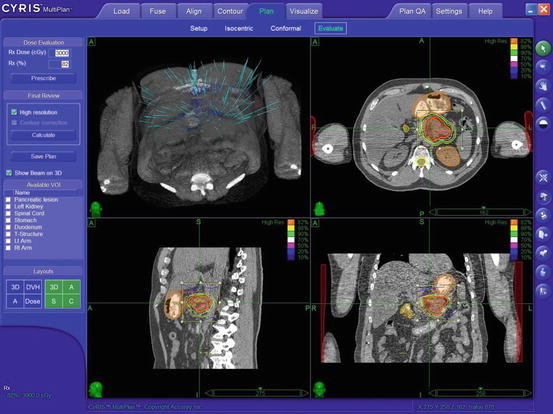
Fig. 10.2
Representative treatment plan
Normal tissue constraints are respected during treatment planning. When treating in three fractions, the volumes of liver receiving 21 Gy (V21) or more and 15 Gy (V15) or more are kept below 30 and 50 %, respectively. The corresponding doses to these volumes in single fraction would be V12 and V 7 [42]. The volume of each kidney receiving 12 Gy or more will be maintained below 25 %. The total maximal spinal cord limit is usually 12 Gy and the maximum point dose to the bowel is less 10 Gy per fraction.
10.6 Treatment Delivery
The CyberKnife Robotic Radiosurgery system (Accuray Incorporated, Sunnyvale, CA) with Synchrony motion tracking has been commonly used as a SBRT system to treat locally advanced pancreas cancer. Other SBRT modalities with image guidance and respiratory gating or dampening (E.g. Novalis, TrueBeam) can be equally applicable to treat these patients. Patients are generally premedicated with H2 receptor blockers or proton pump inhibitors, and antiemetics.
10.7 Systemic Chemotherapy
Patients with unresectable, locally advanced pancreatic cancer often progress with metastatic disease as they harbor micrometastasis at presentation. Hence systemic therapy plays a vital role in the management of these patients. By utilizing a strategy of delivering upfront systemic chemotherapy it is possible to select those patients who are more likely to benefit from local therapy [12, 31]. Intensified chemotherapy regimens with proven improved response rates in the metastatic setting, like FOLFIRINOX, or Gemcitabine and nab-Paclitaxel is now being routinely used in patients with locally advanced pancreas cancer.
The use of single- and multiple-fraction SBRT has been shown to be feasible and safe for patients with locally advanced pancreatic cancer. The results of published data on the use of SBRT in locally advanced pancreas cancer is presented in Table 10.1 [25–27, 29–36, 39, 43]. In contrast to 5–6 weeks of conventional chemoradiation, SBRT can be performed in only 1–3 days, resulting in only a minimal delay in initiating systemic therapy. When SBRT is selectively used for patients who have been treated with systemic therapy, it appears to benefit them most without immediate overt development of metastasis when used upfront [31, 44].
Table 10.1
Clinical outcomes in published literature for SBRT for locally advanced pancreas cancer
Study (author) | Treatment | Number of patients | Progression-free survival (months) | Overall survival (months) |
|---|---|---|---|---|
Koong et al. Phase I [29] | SRS 15–25 Gy | 15 | 2 | 11a |
Koong et al. Phase II [30] | RT 45 Gy + SRS 25 Gy | 19 | 4.5 | 8a |
Schellenberg et al. [26] | GEM + SRS 25 Gy + GEM | 16 | 9 | 11.4a |
Chang et al. (includes all of above patients) [25] | SRS 25 Gy ± GEM EBRT | 77 | – | 11.4a |
Hoyer et al. [27] | SBRT 45 Gy | 22 | 4.8 | 5.7a |
Mahadevan et al. [32] | SBRT 24–36 Gy + GEM | 36 | CA 19–9: 7.9 | 14.3b |
CT: 9.6 | ||||
Polistina et al. [33] | GEM + SBRT 30 Gy | 33 | NR | 10.6a |
Didolkar et al. [43] | SBRT 15–30 Gy + GEM | 85c | NR | 18.6a 8.6b |
Rwigema et al. [34] | SRS 18–25 Gy | 71d | NR | 10.3b |
Mahadevan et al. [31] | GEM- SBRT-GEM | 39 | 15 | 20a |
Goyal et al. [39] | SRS 20–25, SBRT 24–30 (3#) ± Chemo | 20 | NR | 14.4 |
Chuong et al. [36] | GEM/GTX (borderline)-SBRT 35/25 Gy (5#) | 64 (57 borderline) | 9.7 | 15 (16.4 borderline) |
Tozzi et al. [35] | SBRT 36 Gy (6#) | 30 (9 Recc.) | 8 | 11 |
Initial experience with single fraction SBRT with or without external beam radiation has been fraught with acute and chronic toxicity [25, 27, 29, 30]. Similarly high fixed doses of SBRT without accounting for respiratory motion has be associated with significant toxicity [27]. More recently tolerance based moderate doses of hypo fractionated radiation, with respiratory motion tracking and in the setting of systemic therapy has proven to be an acceptable regime [31]. Assuming an α/β ratio of 10 for pancreatic tumor response, Chang et al. calculated their scheme to be equivalent to 74 Gy delivered in 1.8-Gy fractions of conventional radiation. The Hoyer et al. study gave a dose equivalent to 95 Gy. In the study from Mahadevan et al., the equivalent dose was 51–76 Gy, comparable to a conventional radiation dose. While this may potentially appear to decrease the likelihood of local control it likely provides a better therapeutic ratio. The RBE (Relative Biological Effectiveness) and the equivalent doses for tumor control, acute and late toxicity in these series are presented in Table 10.2 [25, 27, 32].
Table 10.2
Relative radiation dose, local control and toxicity in three commonly used prescriptions for stereotactic radiosurgery or stereotactic body radiotherapy
Study | Fractionation | Conventional radiation equivalent dose (Gy) Eq1.8 | Tumor control biological equivalent dose (Gy) BED10 | Long term toxicity equivalent dose (Gy) BED3 | Median follow-up (months) | Local control (%) | Grade 3 or higher toxicity | |
|---|---|---|---|---|---|---|---|---|
Acute (%) | Long term (%) | |||||||
Hoyer et al. [27] | 15 Gy × 3 | 95 | 112.5 | 270 | 6 | 57 | 78 | 33 |
Chang et al. [25] | 25 Gy × 1 | 74 | 87.4 | 233 | 12 | 84 | 1 | 9 |
Mahadevan et al. [32] | 8–12 Gy × 3 | 51–76 | 43–54 | 88–180 | 24 | 78 | 8 | 6 |
10.8 Toxicity
Most patients experience fatigue and some nausea and temporary loss of appetite. Flare pain at the tumour site can also sometime occur and is usually self-limiting. Given the high doses of radiation per fraction, and the close association of the gaswPhase I and Phase II SBRT toxicity data for liver tumors suggest that the maximum point dose to the duodenum should be kept below the equivalent of three fractions each of 10 Gy (equivalent to 130 Gy in 1.8-Gy fractions, assuming an α/β ratio of 3) [40]. Other Dose Volume constraints have been proposed [45, 46]. The acute and long-term toxicity of the Hoyer study was higher than either the Stanford or the Boston groups. Assuming an α/β ratio of 3, the Chang et al., Hoyer et al. and Mahadevan et al. treatment schemes are equivalent to 233 Gy, 270 Gy, and 88–180 Gy in 1.8-Gy fractions, respectively. While this may explain the toxicity, a tolerance based (gastroduodenal tolerance) approach could decrease toxicity. This strategy has been used to reduce toxicity in other cancers treated with SBRT [47, 48].
10.9 Review of Literature
The treatment of patients with non-metastatic locally advanced pancreatic cancer continues to evolve. While the data is conflicting, the general standard of care appears to be concurrent chemoradiation in addition to systemic chemotherapy [49]. However, the effectiveness of the addition of chemoradiation to a chemotherapy treatment plan has been questioned. In addition there are significant side effects associated with 5–6 weeks of upper abdominal radiation, which are particularly problematic for these patients with short life expectancies. Nevertheless, randomized trials have shown a survival benefit to giving radiation therapy to such patients, as in other gastrointestinal cancers, and radiotherapy may be particularly helpful in local control and palliating local symptoms [50–52].
The use of single- and multiple-fraction SBRT has been shown to be feasible and safe for patients with locally advanced pancreatic cancer in several series. In contrast to 5–6 weeks of conventional chemoradiation, SBRT can be performed in only 1–3 days, resulting in only a minimal delay in initiating systemic therapy. Table 10.1 summarizes the outcomes for published studies of SRS and SBRT. Initial experience with single fraction SBRT with or without external beam radiation has been fraught with acute and chronic toxicity. Similarly high fixed doses of SBRT without accounting for respiratory motion has been associated with significant toxicity. More recently tolerance based moderate doses of hypo fractionated radiation, with respiratory motion tracking and in the setting of systemic therapy has proven to be an acceptable regime. While fractionation may decrease the likelihood of local control it potentially provides a better therapeutic ratio. The RBE (Relative Biological Effectiveness) and the equivalent doses for tumor control, acute and late toxicity in these series are presented in Table 10.2. Assuming an α/β ratio of 10 for pancreatic tumor response, 25 Gy single fraction is equivalent to 74 Gy delivered in 1.8-Gy fractions of conventional radiation. Similarly 45 Gy in three fractions delivers a dose equivalent to 95 Gy and the 24–36 Gy equivalent doses are 51–76 Gy, comparable to a conventional radiation dose. This along with consideration of equivalent doses for long-term toxicity as described above may explain the differences in outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree