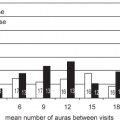
7 There is a consensus that radiosurgery has an important role to play in the management of vascular lesions. Its importance in the management of arteriovenous malformations (AVMs) is well established and discussed elsewhere in this book. It is therefore remarkable that the use of radiosurgery in the management of the other clinically important vascular malformations, namely, cavernoma, is still controversial. Some experts claim that cavernomas respond to radiosurgery just like AVMs, some that radiosurgery can be used as a salvage treatment only, and others that radiosurgery should not be used at all. In general, such diverse opinions are caused by anecdotal reports and limited experience, which leads to extrapolation of results from an insignificant number of cases. It is therefore to be expected that the literature reporting cavernomas responding to radiosurgery is sparse, which is certainly true as compared with the number of publications reporting results following AVM radiosurgery. Another thing to be noticed is that few, if any, experts recommend radiosurgery for cavernomas that have not hemorrhaged, whereas prior hemorrhage has little or no influence on AVM management. If knowledge is limited in regard to how cavernomas respond to radiosurgery, the situation is even less satisfactory following fractionated radiation treatment. For comparison, there are few publications addressing AVM response to fractionated radiation, and the reported experience varies from article to article, making it difficult to draw any definite conclusions. We therefore performed a meta-analysis recently, suggesting that fractionation as compared with radiosurgery offers no or very limited benefit in the management of AVMs.1 It is to be noted that no published information is available on how cavernomas respond to fractionated radiation treatments. One possible conclusion is that the absence of such articles reflects the lack of favorable treatment results, but it is also possible that no group has treated a sufficiently large enough number of patients to allow the researchers to publish their experience. One may argue that cavernomas were difficult to visualize in the era prior to magnetic resonance imaging (MRI) and, consequently, that AVMs, which have been treated with radiosurgery since the 1960s, have had at least a 20-year head start in terms of treatment results. However, cavernomas have been treated based on MR images from the late 1980s, and thus a sufficient number of patients should have been treated to allow conclusions to be drawn about the efficacy of the treatment. In addition, with the increased use of MR scanners, there are more and more cavernomas diagnosed, significantly more than AVMs, making them the most commonly diagnosed vascular malformation. It is therefore not surprising that the interest in managing cavernomas in general, and the role of radiosurgery in particular, has increased. To address that issue, we will summarize, assess, and interpret the published results and also relate them to our own very limited experience. Cavernomas are one of four classically described types of vascular malformations, the others being AVMs, venous malformations, and capillary telangiectasias. A cavernoma is also referred to as a capillary hemangioma, cavernous angioma, cavernous hemangioma, and cavernous malformation, although some researchers suggest that cavernous hemangiomas are a different entity.2 Some believe that the old term angiograpically occult vascular malformation (AOVM), which described an intracerebral hemorrhage where the source of the hemorrhage could not be identified on angiography, equals a cavernoma, but this is not entirely true, as spontaneously obliterated small AVMs were included in this category as well. Cavernomas are discrete, lobulated, and well-circumscribed lesions. They are composed of dilated, thin-walled capillaries that have a simple endothelial lining with variably thin fibrous adventitia indistinguishable from the lining of capillary telangiectasia. They consist of cavernous spaces lined by endothelial cells and collagen. They have no brain parenchyma between the centrally placed vascular channels, and elastic fibers in the walls of the vascular spaces are absent. The arterial supply is rarely visualized.3 The incidence of cavernomas is unknown. Articles from the pre-MRI era suggest a 0.5 to 4.0% incidence.3 With the growing use of MRI, an increasing number of cavernomas are diagnosed, and an incidence of three central cavernomas per million per year was recently suggested.4 There is a hereditary component, and up to 50% of patients with cavernomas have a strong family history that is usually autosomal dominant with incomplete penetration. They may occur throughout the central nervous system, including the spinal cord and cauda equina. Although usually solitary, multiple lesions also occur, especially in the familial cases. It has been suggested that intraparenchymal cavernomas are a different identity as compared with cavernous sinus cavernomas,2 and we will thus address the two types separately. Gonzales et al described a multitude of differences between cavernomas and cavernous sinus hemangiomas (CSHs).2 CSHs are more common in women, whereas cavernomas are not. There is no hereditary component in CSH. Cavernomas are vascular malformations, whereas CSHs grow over time, a behavior consistent with a neoplastic lesion. CSHs do not manifest with acute bleedings, but cause compression symptoms from the cranial nerves. They are high-flow lesions, whereas cavernomas are low flow. They have a capsule or pseudocapsule and show no evidence of previous hemorrhage. They tend to hemorrhage profusely at surgery, and a 36% surgery related morbidity has been reported.5 From a radiosurgical perspective, the above would suggest that CSHs respond to radiosurgery like meningiomas and hemangiopericytomas. Although sparse, the published reports are consistent with this assumption. Shibata and Mori were the first to publish results on the radiation response of a CSH.6 They reported three cases treated with fractionated radiotherapy; all lesions responded favorably and decreased, but the follow-up time was short. Maryishi et al7 confirmed the findings, reporting a decrease in the tumor 8 months after radiotherapy. Other authors have reported similar results following radiosurgery.8–13 The number of cases are few, with each article reporting between 1 and 5 patients, for a total of 16 patients. The observation time varies between 0.5 and 5 years, with a mean time of 2.5 years. The prescription doses vary between 12 and 19 Gy, with a mean value of 15 Gy. Nine patients improved clinically after the treatment, mostly with an improved cranial nerve function. One tumor decreased slightly, 11 significantly, and 4 almost disappeared. No complications were reported. It is interesting to note that all lesions responded in a similar way despite the different doses given; thus, it seems preferable to use lower treatment doses. The only caveat is the relatively short follow-up times, and later tumor recurrence is a possibility that may change the preceding results. Still, CSHs seem to respond favorably to radiosurgery, so well that radiosurgery is probably the treatment of choice for such hemangiomas. There are three reasons to treat cavernomas: for the mass effect in CHSs (as reported above), for the propensity to cause clinically symptomatic hemorrhages, and/or seizures for the intracerebral lesions. This means that the results following CSH radiosurgery are best assessed by MRI. For intracerebral lesions, the aim is to affect the impact of the cavernoma on surrounding brain tissue for patients suffering from epilepsy and to change the natural course of the malformation for the other patients. A very important consequence of the above is that the treatment results in patients with intracerebral cavernomas that cannot be assessed by imaging. Unlike for AVMs, imaging, whether angiography, MRI, or MR angiography (MRA), is therefore of very limited value in evaluating the response to the treatment. For patients with epilepsy, a successful treatment will result in a significant decrease or a cessation of seizures. In regard to a change in the risk for cavernoma rupture, it can only be judged by a comparison to the natural cause of the cavernoma or by histological verification that the cavernoma has been obliterated. Seizures are one of the most common clinical presentations of cavernomas. It is assumed that the epileptic activity from a cavernoma arises not from the lesion itself, but from the surrounding hemosiderin deposit as well as from other degradation products from hemoglobin. Thus, it seems illogical to treat the cavernoma with radiation to affect the surrounding neural tissue. In addition, seizure is one of the complications following radiosurgery, which raises some concern regarding treating epilepsy with radiation. Finally, the dose given to the cavernoma must be low to avoid complications, resulting in low doses to the surrounding neural tissue as well. One can argue that it could be possible to affect the epileptogenic activity without causing tissue necrosis, but our very limited experience at the Karolinska Institute contradicts this assumption. By analyzing the literature, one must conclude that the clinical results published contradict the speculations above. We have found three articles in which the results following radiosurgery for epilepsy associated with cavernomas are presented.14–16 Because two of them reported experiences from the same patient material,14,15 we will focus on the more recent of the two.15 Regis et al and Hsu et al reported results following radiosurgery for cavernomas associated with seizures.15,16 We will concentrate on the patients with intractable epilepsy, here defined as uncontrolled for at least 1 year as well as having a follow-up for at least 1 year after radiosurgery. Regis et al reported on 49 patients, all with intractable epilepsy and all followed for at least 1 year after radiosurgery.15 The prescription doses varied between 15 and 24 Gy, and the follow-up time varied between 11 and 37 months (which contradicts the 1-year minimum follow-up time, although the difference is insignificant). Regis et al found that 26 patients (53%) were seizure free or having an aura only (Engel I), 12 (24%) were almost seizure free (Engel II), two (4%) experienced worthwhile improvement (Engel III), and 11 (22%) showed no worthwhile improvement (Engel IV). They also noticed that the results were inferior for mesiotemporal epilepsy, which makes sense, as the microsurgical results are better if the hippocampus is removed. A caveat from this study is that the complication rate is not reported, and we cannot exclude the possibility that some of the short-term beneficial effects from the radiosurgical treatments are mediated through radiation-induced edema. Thus, follow-up studies over a longer period of time would have been valuable. Hsu et al reported the results from 14 patients in whom the cavernomas causing seizures were treated with radiosurgery.16 In eight patients, the history of seizures was 1 year or less, leaving six patients with intractable epilepsy. The follow-up time was over 1 year for all patients. All six patients improved, three to Engel I, two to Engel II, and one to Engel III. One patient developed an asymptomatic radiation-induced edema. The antiepileptic medication was increased in one patient, making it difficult to assess if the medication or the radiation was responsible for the improvement, but the medication was either unchanged (four patients) or decreased (one patient) for the other five patients. The details of the seizure disorders are usually not included in the publications reporting general treatment results. Some report the time between the onset of seizures and the radiosurgical treatment, and others only note that the patients had pretreatment seizures. We are not informed if the epilepsy is intractable or not for patients with long-lasting seizure problems. Thus, the results from these articles are more nonspecific, as factors other than radiation may contribute to a change in seizure activity. Amin-Hanjani et al reported that 18 patients had seizures before the treatment, 8 with fair and 10 with poor seizure control.17 After the treatment, 1 had no seizures, 13 had fair, and 4 had poor seizure control. Chang et al reported that seizures resolved in two of the four patients with this problem.18 Huang et al19 reported 13 patients with seizures, 5 of whom suffered from seizures for more than a year before the treatment. Three of these patients improved to Engel I and two to Engel III. Among the other patients, five improved to Engel I and three to Engel II. Kida et al20 reported that seven patients had seizures before treatment and that the seizures disappeared in three patients, improved in three cases, and deteriorated in one. Kim et al did not report details about pretreatment seizures but they concluded that 9 of 12 patients were seizure free without medication after the treatment and that the seizures persisted in the other 3 patients.21 Liscak et al reported that 44 patients had seizures before treatment and that improvement occurred in 20 of them.22 They also observed transient seizure deterioration caused by radiation-induced edema in two patients. Liu et al defined patients with a seizure history of longer than 6 months as having chronic epilepsy.22 In the 17 with chronic epilepsy, 4 improved to Engel I, and 13 were reported as Engel III or IV. Stea et al reported that two of the treated patients had experienced seizures.23 One of the patients became seizure free, and one improved after the radiosurgical treatment. However, the latter improved with change in his medication. Tsien et al reported that one patient who had seizures before the treatment became seizure free and remained so 4.5 years later.24 Finally, Zhang et al reported that 33 patients had seizures before the treatment and that follow-up information is available from 31 of them.25 Twenty-two patients improved, and the other nine were unchanged. Zhang et al make an important observation and write that “Six patients whose cavernous hemangiomas were located in the temporal lobe had seizure control when there was manifest brain edema. However, the seizure control deteriorated when the edema resolved.” The conclusion from analyzing the studies above must be that radiosurgery does decrease the epileptic activity from a cavernoma and its surrounding hemoglobin degradation products. In the rest of the chapter we will analyze and assess the risk for hemorrhage of a cavernoma and how it responds to radiosurgical treatment. One way to assess the response to radiation is to analyze the irradiated lesion histopathologically. For AVMs, several reports have shown how they respond to radiation.26–33 In contrast, only two publications have been found analyzing histological postradiation changes in cavernomas. Gewirtz et al analyzed 8 cavernomas that were surgically excised after radiation treatment, radiation being fractionated radiation, Gamma Knife Surgery® (Elekta Instruments AB, Stockholm, Sweden) surgery, or helium ion particle beam.34 These 8 were compared to 10 nonirradiated cavernomas. The mean time between radiation and surgical extirpation was 28 months. Seven of the irradiated lesions showed marked fibrosis of the vascular channels, fibrinoid necrosis, and ferrugination. The fibrinoid necrosis was the only finding unique to the irradiated lesions as compared with the controls. Gewirtz et al conclude: “Our present results suggest that there is little pathological changes that can be ascribed to radiation in these AOVMs. Clearly, the vascular channels remain patent, unlike high-flow arteriovenous malformations, which often sclerose and thrombose close with radiosurgery.” They continue: “Perhaps the lack of an elastic lamina or a muscular layer prevents AOVMs from sclerosing like the high-flow AVMs.” Nyáry et al reported the histopathological analysis of a cavernoma that was excised after having been irradiated with fractionated radiation to a total dose of 40 Gy and removed 1 year later.35 Dose per fraction is not reported. The specimen was compared to nonirradiated cavernoma. The comparison “indicated that there was endothelial cell destruction and marked fibrosis with hyaline degeneration and scar tissue formation in the connective tissue stroma” of the irradiated cavernoma. In addition, most of the vessels were obliterated in the irradiated tissue sampling.” As seen, the two articles contradict each other. One possible explanation for this may be found in the paper by Seo et al.36 They treated 9 cavernomas with radiosurgery, and one of the patients was operated on after the treatment. They write in the abstract that “Histological findings revealed an arteriovenous malformation [AVM] and a cavernous angioma. There was hypertrophy of the internal membrane of the AVM, but no effect on the cavernous malformation.” It seems possible that the case reported by Nyáry et al is similar to this, and thus it is fair to conclude that histopathological verification fails to show that radiosurgery changes the natural course of a cavernoma. To define the efficacy of radiosurgery, we must therefore compare the pre- and posttreatment risk for hemorrhage. Consequently, we must define the risk for hemorrhage from an untreated cavernoma, in other words, the natural course of the disease, before the impact of radiosurgery can be assessed.
Stereotactic Radiosurgery for Cavernomas
Bengt Karlsson and Michael Söderman
 Characteristics and Incidence of Cavernomas
Characteristics and Incidence of Cavernomas
Cavernomas versus Cavernous sinus Cavernomas
 Indications for Radiosurgery
Indications for Radiosurgery
 Cavernomas, Epilepsy, and Radiosurgery
Cavernomas, Epilepsy, and Radiosurgery
 Cavernomas, Hemorrhage, and Radiosurgery
Cavernomas, Hemorrhage, and Radiosurgery
Histopathological Analysis of Radiation Response
 Risk for Hemorrhage from an Untreated Cavernoma
Risk for Hemorrhage from an Untreated Cavernoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree