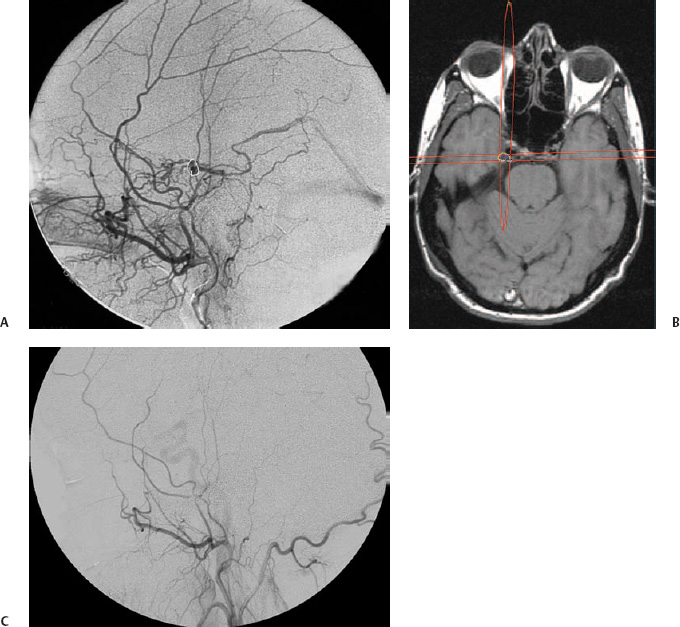
6 In the adult population, intracranial dural arteriovenous fistulas (DAVFs), also called dural arteriovenous shunts or malformations, are about 10 times less frequent than brain arteriovenous malformations (AVMs).1 DAVFs are lesions in the dura mater, acquired in relation to a thrombotic process in the intracranial venous system, most often in a dural sinus.2–5 The symptoms at presentation and the prognosis depend on the location of the arteriovenous fistula, particularly on the venous drainage pattern.6 A DAVF that drains, directly or indirectly, into the cortical venous system may cause venous congestion, leading to intradural hemorrhage or, less commonly, to increased intracranial pressure. The elevated intracranial pressure can cause nonfocal neurologic symptoms such as dementia. Patients at risk for bleeding or other adverse events are usually offered treatment.7–10 Intolerable bruit or significant exophthalmos or chemosis related to increased pressure in the superior ophthalmic vein may also prompt treatment. Microsurgery and embolization are established treatment options for DAVFs, and the indications and methods have been well described elsewhere.10,11 Irradiation as a means of DAVF treatment has been proposed by several groups. There are several reports on the use of fractionated irradiation (i.e., various forms of radiotherapy).12–16 However, radiosurgery, initially with a single cobalt 60 source and subsequently with the Gamma Knife (Elekta Instruments AB, Stockholm, Sweden), seems to have emerged as the preferred method.17–22 There are very few reports on linear accelerator radiosurgery of DAVFs.23–25 DAVFs are acquired abnormal arteriovenous connections within the dura mater.26 The blood supply is from meningeal arteries, albeit a minor contribution from pial arteries is not uncommon. The venous exit is directly to a dural sinus, to a leptomeningeal vein contiguous with the dura mater, or both. DAVFs in the cavernous sinus most often empty into the superior ophthalmic vein. The pathophysiology of spontaneous DAVFs seems to be related to venous thrombosis and venous hypertension, both prior to and after the emergence of the shunt.27,28 In this disease there is upregulation of vascular endothelial growth factor as well as basic fibroblast growth factor, epinephrine B2, and hypoxia-inducible factor 1 in rat models with induced intracranial venous hypertension.29–32 A similar pattern of growth factor upregulation has been found in operative specimens and blood samples from patients with DAVFs.33–36 The presentation and relevance of a DAVF depend on the location of the shunt and on the venous drainage pattern.6,37–42 Widely used classification systems mix anatomical location and venous drainage but can be useful for descriptive purposes.43–45 However, it is principally the venous drainage pattern that determines the prognosis.3,46 Thus, a DAVF without cortical venous drainage or reflux may present with externally audible pulse-synchronous bruit or symptoms related to orbital venous congestion, but it will not cause a hemorrhage or elevated intracranial pressure. Not infrequently it is an incidental finding during a neuroradiological investigation performed for an unrelated reason.8,47 Lesions localized in the wall of the cavernous sinus regularly drain through the superior ophthalmic vein, causing orbital venous congestion visible as chemosis and exophthalmos. The risk for intracranial hemorrhage is elevated if the blood is not redirected into the cortical venous system, directly or indirectly. In this context it should be noted that some patients use several venous pathways to drain a DAVF and that drainage through a dural sinus or the superior orbital vein may coexist with leptomeningeal drainage; consequently, the patient is at risk for intracranial hemorrhage. Also, a change in symptoms in a fistula without cortical venous reflux, including disappearance of a symptom like bruit, may indicate rerouting of the venous drainage, potentially into cortical veins, converting a “benign” fistula into a dangerous one. Such changes, therefore, should always warrant an angiographic investigation. A DAVF with cortical venous reflux may present with bruit or chemosis, but also with neurologic deficits, frequently because of intracranial hemorrhage. The data on the natural course of DAVFs are limited and ambiguous, to say the least. Brown et al found an annual incidence of hemorrhage of 1.8% in all patients with DAVFs and 3% among those with drainage into leptomeningeal veins.47 In this series, all the patients that bled had leptomeningeal drainage with a venous varix. Duffau et al analyzed the early outcome of 20 consecutive patients and found a 35% rebleeding rate within 2 weeks of the first hemorrhage.7 In 2002 van Dijk et al reported an annual hemorrhage rate of 8% and an annual mortality rate of 10%9 from DAVFs with long-standing cortical venous drainage. In the most recent and largest series, the annual risk of hemorrhage was 7.4% in patients who presented with intracranial hemorrhage and 1.5% in those who did not. There was no mortality.8 To summarize, it may very well be that the annual risk of hemorrhage from a nonruptured DAVF is <2%, whereas it is ~7% if it presents with bleeding. In this context, the angioarchitecture of the lesion should probably also be taken into consideration, but more data are needed. In a patient with limited symptoms and without cortical venous drainage from a DAVF, abstention from treatment is clearly a management option, particularly in view of the relatively high likelihood of spontaneous occlusion or symptom regress.6,9,47,48 However, these patients should be monitored both clinically and radiologically, as a benign DAVF may in rare instances develop leptomeningeal venous drainage.49 A change in symptoms, even an improvement (e.g., a reduced bruit), should arouse suspicion of conversion that warrants an angiographical investigation. Patients harboring a DAVF with cortical venous drainage, and therefore at risk for intracranial hemorrhage or nonfocal neurologic deficits, are usually offered treatment.7–10,46 In this patient group, radiosurgery is a treatment alternative if embolization or surgery is considered unacceptably dangerous or impossible. Patients with hemorrhage as the presenting event and those with a focal leptomeningeal venous dilatation may be at considerable risk for bleeding during the time before the DAVF obliterates and should be subjected to radiosurgery only if there is no other realistic alternative.8,47 Patients with intolerable bruit from a DAVF without cortical venous drainage can be treated with radiosurgery if they accept the latency period and the fact that not all patients will be cured. Significant exophthalmos or chemosis secondary to a DAVF in the cavernous sinus should also prompt an active approach, particularly because visual impairment secondary to the increased intraorbital pressure has been reported.47 These patients can usually be cured with transvenous embolization, mostly with detachable coils, of the cavernous sinus through a skull base sinus or occasionally through the superior ophthalmic vein. Transarterial embolization with liquid adhesive in this region requires precise knowledge of the individual patient’s anatomy, and in view of the possible complications, it should not be taken on lightly.50 Transarterial particle embolization with the aim to alleviate symptoms and speed up the obliteration process has been attempted.51 However, some patients have severe symptoms from a cavernous sinus DAVF that is inaccessible for embolization and not amenable to surgery, whereas others have relatively little orbital congestion. Both of these patient groups could be offered treatment with radiosurgery. Prior to radiosurgery, stereotactic angiography is required to define the target, that is, the arteriovenous shunting zone, because this zone can seldom be adequately located using magnetic resonance imaging (MRI) or computed tomography (CT) alone (Fig. 6.1A). The angiographic technique is very similar to that previously described for radiosurgery for brain AVMs.52 The initial target volume is projected onto cross-sectional stereotactic imaging, usually MRI, so that radiation doses to sensitive tissue such as the skin, the brainstem, and the cranial nerves can be calculated and kept acceptably low (Fig. 6.1B).20 In DAVFs of the cavernous sinus, the optic apparatus is of particular concern because of its low tolerance for ionizing radiation. Traditionally, the maximum radiation dose to the optic pathways has been 8 Gy; however, recent data indicate that the optic nerve can probably tolerate higher radiation doses, up to 11 Gy, over a short distance with a low risk of radiation-induced optic neuritis.53,54 The arteriovenous shunt is located in the dura, most often in the wall of a dural sinus, but it may extend into the previous lumen in the presence of a neovascularization within an old thrombosis. Gadolinium-enhanced T1-weighted images in axial and coronal planes are helpful to identify the dura mater and the sinus. DAVFs are treated with radiation doses similar to those in radiosurgery for brain AVMs.22 Thus, lesions remote from cranial nerves and other radiation-sensitive tissue are irradiated with 25 Gy to the 40 to 60% isodose. Dose plans are adjusted according to the radiation sensitivity of adjacent structures (Fig. 6.2). Using the Gamma Knife, collimator plugging with the aim to reduce skin radiation doses is frequent in these often superficial targets. The treatment volume is usually below 2 mL, and radiation doses therefore do not have to be reduced below 25 Gy to decrease the risk for an adverse radiation reaction in the adjacent brain. Thus, volume is generally not a limiting factor, particularly because some of the structures in the periphery of the target are often relatively insensitive to radiation, such as bone and dural sinus. Fig. 6.1 Dural arteriovenous fistula (DAVF) in the medial wall of the middle fossa. The patient had suffered from pulse-synchronous bruit for about 6 months before she was first examined. She had not bled. The bruit disappeared 8 weeks after Gamma Knife radiosurgery. (A) Stereotactic angiography. Lateral view with injection into the external carotid artery. Note the drainage into the basal vein of Rosenthal. The target volume is delineated. Treatment was performed with 25 Gy to the periphery of the lesion. (B)
Stereotactic Radiosurgery for Dural Arteriovenous Fistulas
Michael Söderman, Bengt Karlsson, Bodo Lippitz, and Tommy Andersson
 Pathophysiology of DAVFs
Pathophysiology of DAVFs
 Clinical Presentation and Natural History of DAVF
Clinical Presentation and Natural History of DAVF
 Indications for Radiosurgery
Indications for Radiosurgery
 Radiosurgical Technique
Radiosurgical Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Stereotactic Radiosurgery for Dural Arteriovenous Fistulas