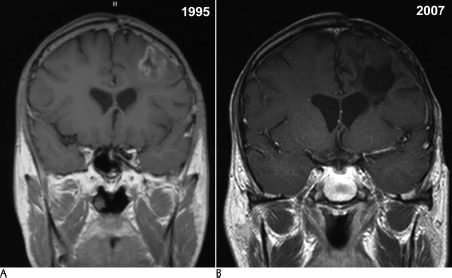
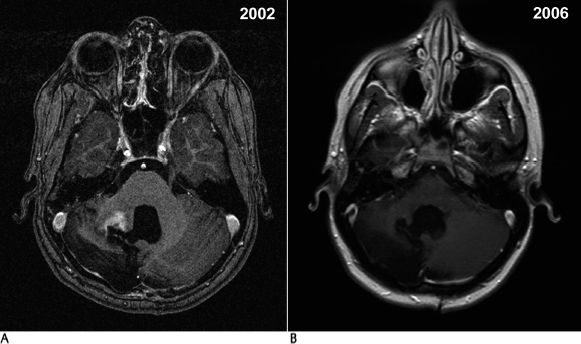
18 The management of patients diagnosed with gliomas is wide ranging. Depending on the primary pathology of the tumor, a combination of surgical resection, therapeutic radiation, and chemotherapy can be used for treatment.1–6 With the advent of radiosurgery, neuro-oncologists have another tool to accurately and safely treat intracranial pathology. Unlike conventional radiotherapy, stereotactic radiosurgery allows for very high doses of radiation to be delivered to a small volume of tissue with very little dose to surrounding tissue. This feature makes it ideal for any lesion that is readily discernible from normal structures on imaging; thus, the majority of experience has been with extra-axial lesions, such as meningiomas,7 acoustic neuromas,8 and pituitary tumors,9 or with intra-axial lesions that have readily discernible structures, such as arteriovenous malformations.10 The only intra-axial tumors that have been extensively treated with stereotactic radiosurgery are brain metastases. Metastases are different from gliomas in that they are usually distinct, contrast-enhancing lesions that are much less likely to infiltrate surrounding brain tissue.11 Stereotactic radiosurgery also allows for doses of radiation that make radiosensitivity of tumors much less of an issue. Gliomas are by nature more infiltrative and are more difficult to target as separate from normal brain; thus, stereotactic radiosurgery for these lesions would not seem optimal. However, in the case of malignant gliomas, not only is radiation therapy the most effective management tool, but overall survival appears to be correlated with the total dose delivered, up to a total dose of 60 Gy.12,13 Also, the majority of tumor recurrences occur within 2 cm of the enhancing margin of the original tumor, leading to the theory that increasing dose to this area may allow for higher local dose of radiation, and improve survival and progression-free survival.14,15 This realization led to the initial interest in and use of interstitial brachytherapy for the treatment of malignant gliomas.16 In this technique, radioisotopes, usually iodine 125, are implanted directly into the tumor cavity after resection to provide maximum radiation dose to the surrounding tissue. Early evidence in the 1990s appeared to show that brachytherapy increased survival rates in patients with brain tumors.16 However, since that time, three separate clinical trials, most recently in 2007, have shown no evidence of a survival benefit but an increased risk of radiation necrosis, toxicity, and reoperations,17–19 and it became clear that selection bias led to the erroneous conclusion that brachytherapy had a positive treatment effect. Despite the hazards of selection bias, several reports describe the use of radiosurgery in the treatment of glial neoplasms. In this chapter we review this experience, by pathologic category. Most of the reported experience with stereotactic radiosurgery to treat gliomas has been for patients with these tumors. In the 1990s, some reports appeared to show good efficacy and increased survival benefit with the use of stereotactic radiosurgery at the time of surgical resection in conjunction with conventional radiotherapy and chemotherapy20–49 (Table 18.1). Similar to the experience with brachytherapy, selection bias is likely to have been the driving force behind the apparent efficacy of stereotactic radiosurgery in these early reviews.50 In an attempt to address the problem of selection bias, the Radiation Therapy Oncology Group (RTOG) 93–05 protocol was established for a randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy and carmustine (BCNU) chemotherapy versus conventional radiotherapy and BCNU chemotherapy alone.43 A total of 203 patients with supratentorial glioblastoma multiforme (GBM) <40 mm were randomized to receive stereotactic radiosurgery plus radiotherapy (60 Gy) and BCNU or radiotherapy and BCNU alone (Fig. 18.1). Stereotactic radiosurgery dose ranged from 15 to 24 Gy depending on the tumor size. At a median follow-up of 61 months, patients in the stereotactic radiosurgery group had a median survival time of 13.5 months versus 13.6 months in the conventional treatment group. The data were also analyzed to see if there was a tail effect, leading to more long-term survivors, but 2- and 3-year survival rates were not significantly different. Recurrence patterns, cognitive decline, quality of life, and type of stereotactic radiosurgery used (Gamma Knife [Elekta Instruments AB, Stockholm, Sweden] versus linear accelerator [linac]) were also analyzed, but the groups did not have significant differences in these areas. The results of this trial proved that upfront stereotactic radiosurgery is not of benefit for patient survival or quality of life, and prior reports of efficacy were likely due to selection bias. Subsequently, in 2005, the American Society for Therapeutic Radiology and Oncology (ASTRO) published an evidence-based review of the role of stereotactic radiosurgery for malignant glioma.44 In this review, the previously mentioned randomized trial was reviewed, along with five prospective20,21,23,29,31 and seven retrospective studies.26,28,30,33,34,36,37 These studies did not have significant improvement in survival, quality of life, or tumor progression. Toxicity did not appear to be increased as a result of the therapy. Overall, there was no evidence that stereotactic radiosurgery was of benefit as an upfront treatment. A second portion of the review concentrated on stereotactic radiosurgery as salvage therapy for recurrent or progressive malignant gliomas. There have been no randomized trials to address this issue. There were three prospective22,27,39 and four retrospective24,30,35,40 studies that tried to assess it. These studies did not demonstrate a survival benefit for stereotactic radiosurgery, and none of them addressed quality of life issues. Recurrence patterns on these patients also did not change. As a result, stereotactic radiosurgery was not recommended as a salvage treatment. Fig. 18.1 (A) A 39-year-old man following resection of a left frontal glioblastoma multiforme in April 1994, followed by external radiation, hydrea, and procarbazine, lomustine, and vincristine (PCV) chemotherapy. He underwent radiosurgical boost of 1000 Gy to the tumor bed in March 1995. (B) Magnetic resonance imaging from September 2007 shows complete resolution of the enhancing tumor and moderate encephalomalacic defect. Multiple other retrospective studies have been published on the subject of stereotactic radiosurgery for malignant gliomas. The University of Florida experience46 with linac stereotactic radiosurgery for malignant gliomas, which was updated in 2005, reviewed a total of 100 patients. These patients were then compared with historical controls from the RTOG’s recursive portioning categories, in an attempt to eliminate selection bias. Sixty-seven of these patients received upfront stereotactic radiosurgery. The remainder had stereotactic radiosurgery as a treatment boost at the time of recurrence or progression. Overall, survival rates were similar to historical controls except in those patients in recursive partitioning analysis (RPA) class V, where the median survival time was 15.6 months for the stereotactic radiosurgery group versus 8.9 months for the historical control. Of course, this study was limited by selection bias, as it was a retrospective review. Hsieh et al47 published results of 51 patients treated with Gamma Knife stereotactic radiosurgery either upfront (25 patients) or at the time of progression (26 patients). They found that those patients treated at the time of progression had increased survival time (16.7 months vs 10 months), but this did not reach statistical significance (p = .09). The authors concluded, however, that there was likely a benefit to stereotactic radiosurgery for patients treated at the time of recurrence. Combs et al48 published their results for linac salvage treatment without adjuvant chemotherapy in 32 patients with GBM. The median interval from primary treatment to stereotactic radiosurgery was 10 months, and the median overall survival time after stereotactic radiosurgery was 10 months. Progression-free survival time was 7 months. This review compared these results to those of chemotherapy alone, with a mean survival time of 5 months. Again, this study was likely limited by selection bias of those patients who had already survived with continued good neurologic function. Overall, stereotactic radiosurgery appears to be another treatment option for malignant gliomas, but further randomized, controlled studies of stereotactic radiosurgery at the time of recurrence of these tumors are indicated to answer the question of whether or not there is benefit to stereotactic radiosurgery. As is clear from RTOG 93–05, upfront stereotactic radiosurgery is not of benefit and cannot be recommended as a treatment option at this time. The safety of stereotactic radiosurgery has also not been proven. Lesion enlargement after stereotactic radiosurgery can be due to tumor recurrence, radiation necrosis, or both. Without tissue diagnosis, it is impossible to determine the true incidence of symptomatic radiation necrosis. It is likely that radionecrosis is underreported. Low-grade gliomas include pilocytic astrocytomas, grade II astrocytomas, oligoastrocytomas, oligodendrogliomas, and, less commonly, glial neoplasms, such as pleomorphic xanthoastrocytoma, ganglioglioma, and subependymoma. Of these tumors, pilocytic astrocytoma and grade II astrocytoma have been most frequently treated with stereotactic radiosurgery. These two tumor types are somewhat distinct in terms of their clinical course. Pilocytic astrocytomas are most common in pediatric patients, are often surgically curable with total resection, and behave in a slow-growing, nonaggressive fashion. Ten-year survival rates for pilocytic astrocytomas are up to 94%. The location of these tumors in unresectable areas such as the hypothalamus, thalamus, optic tract, pons, and cerebellar peduncle can, however, make other treatment options more attractive (Fig. 18.2).4 Grade II astrocytomas are more likely to occur in young adults and have a more aggressive course, with recurrence and malignant transformation leading to a median survival time of 7 to 10 years. These lesions can also have focal areas that are difficult to access with open surgery.2,3 Treatment of these tumor types with stereotactic radiosurgery has largely been for residual lesions after resection or biopsy or for focal areas of nodular recurrence. Fig. 18.2 (A) A 13-year-old female patient status postresection of pilocytic astrocytoma in January 2002, with recurrence on imaging that enlarged over 6 months postoperatively. (B) The patient was treated with 1500 Gy to the 70% isodose line in December 2002 and has no residual effect evident on follow-up in August 2006. There are no randomized trials of stereotactic radiosurgery for low-grade gliomas, but there are some retrospective reviews of patients who were treated with stereotactic radiosurgery as part of a multimodality approach (Table 18.2).40,51–61 Kida et al53 reported on 51 patients treated over an 8-year period. Twelve patients had pilocytic astrocytomas, and 39 had grade II astrocytomas. Of those with pilocytic tumors, after a mean follow-up of 25 months, one patient had complete radiographic resolution of tumor, five had partial reduction in volume, one was slightly reduced in volume, four were unchanged, and one increased in size, for a control rate of 91.7%. For grade II lesions, at a mean of 28.5 months, 5 had complete resolution, 13 had partial reduction, 6 had slight reduction, 10 were unchanged, and 5 increased in size, for a control rate of 87.2%. Forty-one percent of these patients developed radiation edema, and half of these required 2 to 3 months of steroid medication for symptom management. There did not appear to be any long-term complications. Hadjipanayis et al57 reported on 49 patients treated with Gamma Knife stereotactic radiosurgery over a 13-year period. Thirty-seven patients had pilocytic astrocytomas, and 12 had grade II astrocytomas. All diagnoses were confirmed with tissue sampling. Of those treated, 11 had complete tumor resolution, 12 had reduced tumor volume, 10 had stable tumor volume, and 16 had progression. At a median of 32 months after stereotactic radiosurgery and 63 months after diagnosis, 45 of 49 patients were alive, with 3 deaths due to tumor progression. Heppner at al59
Stereotactic Radiosurgery for Gliomas
Gregory J. A. Murad and William A. Friedman
 Anaplastic Astrocytomas and Glioblastomas
Anaplastic Astrocytomas and Glioblastomas
 Low-Grade Gliomas
Low-Grade Gliomas
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree