8 Meningiomas are common intracranial tumors that pose a variety of therapeutic challenges because of brain location, size, histologic subtype, and patient characteristics. Over time, craniotomy and tumor resection along with its dural base became the preferred approach for the majority of symptomatic patients.1 The morbidity of resection was reduced with microneurosurgical technique, better anesthesia, neuronavigation, and improved medical care. However, because these usually benign tumors may be associated closely with critical neural or vascular structures, complete resection may not be feasible.2–5 Meningiomas adjacent to venous sinuses may be resectable only at the risk of major neurologic deficits caused by venous injury. Elderly or infirm patients often sought alternative approaches. In this chapter we review our experience in the care of over 1000 patients with intracranial meningiomas. We have found that stereotactic radiosurgery safely provided high rates of tumor growth control or regression in patients with small to medium-sized benign tumors. Over a 20-year period, we performed radiosurgery on 1191 intracranial meningiomas. We recently reviewed the results from the first 972 patients with 1045 tumors managed over the initial 18 years. The brain locations of these tumors are shown in Table 8.1. The decision to perform radiosurgery was made in patients with residual or recurrent smaller volume tumors after prior resection, those with symptomatic primary tumors in locations associated with higher risk for resection, those with concomitant medical illnesses or advanced age, in younger patients who chose radiosurgery over other available options, and in younger patients with minimal symptoms or who were asymptomatic but chose against observation. Contraindications or exclusion criteria included large tumor volume (mean diameter > 3.5 cm), tumors with symptomatic optic nerve or chiasmal compression, optic nerve sheath tumors with preserved vision, elderly patients with asymptomatic tumors, and tumors with atypical imaging features and no prior histologic diagnosis. The detailed results from 982 tumors (94%) were available for analysis. Five hundred and four patients (51%) had no prior treatment. Prior surgical resections were usually partial removals (84%). A solitary tumor was present in 818 patients and multiple tumors in 161. Twenty-eight patients had neurofibromatosis type 2. Tumor pathology was known in 511 residual or recurrent tumors. Prior radiotherapy had been delivered to 54 patients (48 after a resection, and 6 had radiotherapy alone); 2 patients had prior radiosurgery elsewhere. Eight patients had received chemotherapy. Twenty-five tumors (2.5%) developed after prior fractionated irradiation.
Stereotactic Radiosurgery for Meningiomas
Douglas Kondziolka, David Mathieu, Ricky Madhok, John C. Flickinger, and L. Dade Lunsford
 Patient Characteristics
Patient Characteristics
| Location | Number of Tumors |
| Posterior fossa | |
| Petroclival | 122 |
| Petrous ridge | 66 |
| Foramen magnum | 22 |
| Other | 42 |
| Middle fossa | |
| Cavernous sinus | 306 |
| Sphenoid wing | 32 |
| Other | 13 |
| Anterior fossa | |
| Olfactory groove | 29 |
| Planum sphenoidale | 29 |
| Anterior clinoid | 17 |
| Parasellar | 13 |
| Convexity | 126 |
| Other | |
| Parasagittal | 113 |
| Tentorial notch | 40 |
| Torcular | 6 |
| Falcine | 47 |
| Intraorbital | 13 |
| Intraventricular | 9 |
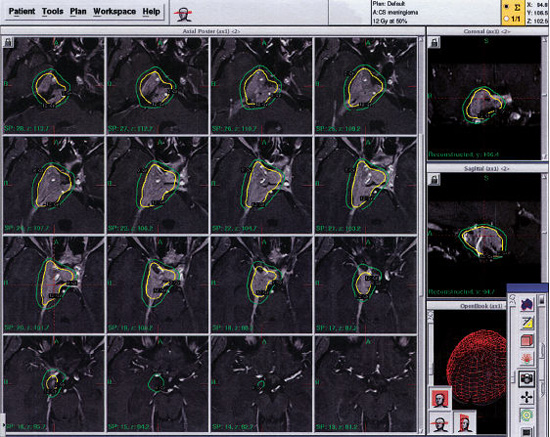
Fig. 8.1 Gamma Knife radiosurgery plan for a 43-year-old woman with a right cavernous sinus meningioma who presented with diplopia. The tumor was planned with 24 isocenters using the Perfexion model Gamma Knife (4, 8, and 16 mm collimators) to deliver a margin dose of 12 Gy and a maximum dose of 24 Gy.
Radiosurgery was performed under local anesthesia with mild sedation as necessary, using a Leksell Gamma Knife (Elekta Instruments AB, Stockholm, Sweden). We have used the Gamma Knife models U, B, C, 4C, and Perfexion for our patients with meningiomas. Radiosurgery was targeted with stereotactic computed tomography (CT) guidance (before 1992) and with magnetic resonance imaging (MRI) since. A mean of 7.5 isocenters were used to provide conformal radiosurgery. The dose received by adjacent critical structures was determined and selective beam blocking used if necessary to restrict the dose fall-off (Fig. 8.1). We delivered a mean dose to the tumor margin of 14 Gy and a mean maximum dose of 28 Gy. The mean tumor volume in this series was 7.4 mL. The mean volume receiving ≥ 12 Gy was 8.4 mL. Radiation doses were prescribed to the 50% isodose volume for 886 tumors (85%). For tumors near the optic nerve or chiasm, the average maximal optic dose was 6.4 Gy. After radiosurgery, patients were discharged home within 24 hours, and in recent years on the same day. The median follow-up in this study was 4 years; 842 patients were still living (86%). Follow-up past 5, 7, 10, and 12 years was obtained in 327, 190, 90, and 41 patients, respectively.
 Tumor Response to Radiosurgery
Tumor Response to Radiosurgery
Imaging studies after radiosurgery showed that 407 tumors had regressed, 454 were unchanged, and 96 had enlarged, for a raw tumor control rate of 90%. Imaging and clinical follow-up were requested for all patients, but they were not complete for all.
Adjuvant Radiosurgery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree