20
Stereotactic Radiosurgery for Pediatric Brain Tumors
Simon S. Lo, Gene H. Barnett, and John H. Suh
Brain tumors constitute 20% of all pediatric cancers and are the most common solid tumors in childhood.1,2 Surgical resection is usually the mainstay of treatment, and maximal safe resection is the goal in most cases. Conventional fractionated radiation therapy is indicated for high-grade or incompletely resected or unresectable tumors.
Stereotactic radiosurgery (SRS) refers to a strategically delivered high dose of radiation to a target volume using megavoltage photons generated by a linear accelerator (linac), gamma rays from a Gamma Knife (Elekta Instruments AB, Stockholm, Sweden) unit, or protons generated by a cyclotron or synchrotron unit,1,2 typically performed in one, but up to five, session(s). SRS has been used extensively to treat brain tumors in adults and has become one of the standard treatments for certain tumors. There is a moderate amount of literature on the use of SRS in the treatment of pediatric brain tumors. Unlike adult patients, there are substantial concerns of radiation-induced damage to the brain, leading to subsequent neurocognitive, neurologic, and neuroendocrine dysfunction. As a result of its favorable dosimetric characteristics, SRS represents a very attractive treatment modality for some pediatric patients with brain tumors. The physics and radiobiology of SRS are covered in other chapters of this textbook. This chapter will focus on the clinical applications and results of SRS in the treatment of pediatric brain tumors.
 Technical Issues Specific to Stereotactic Radiosurgery in Pediatric Patients
Technical Issues Specific to Stereotactic Radiosurgery in Pediatric Patients
Unless a frameless radiosurgical system is used, rigid fixation is typically required in SRS because little or no margin around the target volume is given in treatment planning. As opposed to adults, where rigid fixation of the skull is readily achievable, younger children have thin skulls, which may not be completely fused. An alternative to insertion of pins into the outer skull table is the fabrication of a cast or aquaplast with piers where the pins can be inserted2 (Fig. 20.1). A body cast for neck support is necessary because these patients are under general anesthesia, and their body sizes are small.
General anesthesia is generally required for younger children and older children who are psychologically too immature to withstand the whole radiosurgical procedure, because the patient needs to remain still during head frame placement, acquisition of computed tomography (CT) or magnetic resonance (MR) images, and treatment delivery.1,2 SRS is typically a 1-day procedure that entails lengthy processes mentioned above, complex treatment planning, and transportation of the patient to different parts of the hospital and sometimes a different hospital. Depending on the complexity of the planning and the treatment-planning system used, the time required can vary significantly. The treatment time can be prolonged, especially in circumstances where a Gamma Knife unit with old sources is used to deliver the radiation using trunnions and multiple small shots (isocenters). Prolonged anesthesia of those children undergoing SRS can be expected. To avoid unnecessary radiation exposure to support staff in the treatment area, patients are usually monitored by the anesthesiologist in the control area. When treatment planning is performed, the neurosurgeon and radiation oncologist involved in SRS should strike a balance between the time required in treatment planning and delivery and the extra conformality achieved with more complex planning. A new model of Gamma Knife, the Perfexion (Elekta), allows for shortened treatment and planning times. Radiation dose sculpting is also facilitated. These features may be beneficial for pediatric patients receiving SRS.
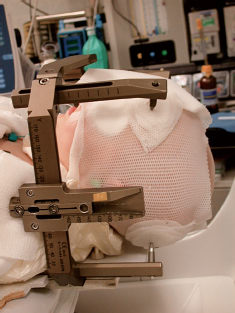
Fig. 20.1 An aquaplast was fabricated with piers where the pins can be inserted for fixation. (From Witt TC, Lo SS, Timmerman RD. Successful treatment of a skull base malignant rhabdoid tumor with surgery, chemotherapy and Gamma Knife-based stereotactic radiosurgery in a young child. Stereotact Funct Neurosurg 2007;85:310–313, with permission from Karger AG, Basel.)
A frameless robotically controlled radiosurgical system such as CyberKnife (Accuray, Inc., Sunnyvale, CA) can potentially solve some of the problems associated with frame-based radiosurgical systems. The feasibility, safety, and efficacy of the use of this system in the treatment of brain tumors in pediatric patients, including very young children, have been demonstrated.3
 Specific Tumors
Specific Tumors
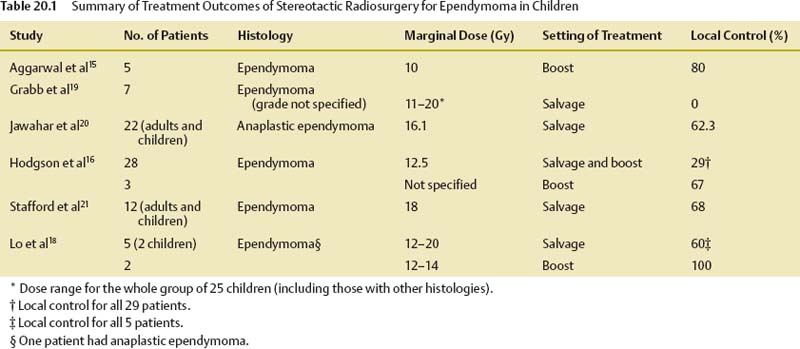
Ependymomas
Ependymomas account for 5 to 10% of all pediatric brain tumors.4 Histologically, ependymomas are well-delineated, moderately cellular gliomas that have a sharp demarcation from the surrounding brain parenchyma.5 Because of this characteristic, they are very well suited for SRS, where the prescribed isodose conforms highly to the target volume, and there is a rapid fall-off of the radiation dose beyond the target volume. Although not specifically categorized by Larson et al, based on their characteristics, ependymomas can be regarded as class IV targets (tumor composed of early-responding tissue with no intermingling normal brain tissue).6
The most important factor that determines survival for patients with ependymomas is the extent of surgical resection.4,7–11 The presence of gross disease predicts a poor prognosis. SRS is used in this disease in children mainly in three settings, namely, as a boost after external beam radiation therapy, as a salvage treatment of recurrent disease, and, less commonly, as the sole treatment.
Boost Therapy
In pediatric patients with ependymomas treated in the primary setting, a radiation dose response has been demonstrated.12–14 This is particularly relevant in patients with high-risk disease (incompletely resected tumor). In a Pediatric Oncology Group (POG) 9132 study, in which radiation dose escalation to 69.6 Gy was achieved by hyperfractionation, the 19 patients who underwent subtotal resection had superior survival outcomes compared with historic controls from an earlier POG 8532 trial.4 SRS can deliver a single focused high dose of radiation to a target volume and has been used as a boost therapy after external beam radiation therapy for high-risk patients.
Investigators from St. Jude Children’s Research Hospital reported their early experience with this approach. Five pediatric patients with residual enhancing ependymoma measuring ≤ 3 cm received a boost with linac-based SRS after external beam radiation therapy to doses of 50.4 to 55.8 Gy.15 The SRS dose was 10 Gy (range 9–15 Gy) prescribed to the 80% isodose line. With a median follow-up time of 24 months (range 15–40 months), 80% (four of five patients) survived with no evidence of tumor progression.15Complete response was achieved in three (60%) of the five patients. Radionecrosis occurred in one (20%) of the five patients. In a pediatric SRS series from Harvard University, 3 of the 28 patients with ependymoma treated with SRS received it as a boost therapy after external beam radiation therapy. Two (67%) of the three patients survived without evidence of disease progression at 30 and 62 months, respectively.16 In a series of nine patients treated with SRS for ependymoma from Washington University, three were treated with SRS as a boost therapy after external beam radiation therapy to doses of 45.0 to 53.6 Gy.17 With follow-up times ranging from 56 to 91 months, the relapse-free survival rate was 100%.17 Two (67%) of the three patients developed complications (seizures in one patient and facial nerve palsy in the other) after SRS. In the series from Indiana University, two pediatric patients were treated with SRS as boost therapy after external beam radiation therapy to doses of 54.0 to 55.8 Gy. Both patients remained progression free at 40 and 65 months, respectively.18 One of the two patients developed radionecrosis, which was controlled with hyperbaric oxygen and steroid therapy.
In summary, the combination of external beam radiation therapy and boost with SRS is associated with excellent local control of gross residual disease in ependymoma, although some patients developed late radiation complications. Out of the 13 patients treated with this approach in the literature, 11 (85%) achieved long-term progression-free survival. However, prospective data supporting this approach are lacking. A prior POG phase II trial involving the use of SRS as a boost therapy for pediatric patients with high-risk ependymoma was closed, secondary to poor accrual. Future trials testing this approach may be worthwhile.
Salvage Therapy for Recurrent Disease
The recommended treatment for patients with recurrent ependymoma after prior surgery and postoperative external beam radiation therapy is salvage surgical resection. However, a complete resection may not be safely achievable in some cases. The response to chemotherapy is usually suboptimal in ependymoma. SRS provides a salvage option for patients where a complete resection is not possible and has been used for the treatment of recurrent ependymoma in pediatric patients.
In an early series from the University of Pittsburgh, seven pediatric patients with recurrent ependymoma (grade not specified) were treated with Gamma Knife radiosurgery. The reported recurrence rate was 100%, and the time to progression was 6 months.19 In a subsequent study from the same institution, 22 patients (including adults and children) with progressive or recurrent anaplastic ependymoma were treated with Gamma Knife radiosurgery. The median dose was 16.1 Gy (range 10–20 Gy). With a median follow-up of 21 months, the 3-year local and cranial control rates were 62.3 and 32.4%, respectively.20 Hodgson et al from Harvard University reported that among the 28 pediatric patients treated with SRS for ependymoma (3 treated with SRS as boost therapy after external beam radiation therapy and the remaining treated for recurrent disease), the median progression-free survival time was 8.5 months, and the 3-year local control was only 29%.16 Out of the 25 patients treated for recurrent disease, only 3 remained disease free. In a series from the Mayo Clinic, 12 adult and pediatric patients with 16 recurrent ependymomas were treated with Gamma Knife radiosurgery. Eleven of them had prior external beam radiation therapy. The median prescribed dose was 18 Gy (range 12–24 Gy). With a median follow-up of 22.5 months, the 3-year local control was 68%, and 2 (17%) of the 12 patients developed distant neuraxis failure.21 Two (17%) patients developed complications. In the Indiana University series, five patients (two children) with 10 recurrent ependymomas were treated with Gamma Knife radiosurgery. Out of the five patients who received SRS as salvage treatment, three (60%) were alive, two (40%) were alive without recurrence, two (40%) developed distant failure, and three (60%) had in-field control.18 The 3-year in-field control for the 10 recurrent tumors was 62.5%.18
Most children with recurrent ependymomas have a prior history of external beam radiation therapy. If a complete surgical resection of the gross tumor is not achievable, they are left without other effective options. Chemotherapy has been used in ependymomas in various settings but with limited success. Local tumor progression is uniformly fatal. SRS provides a minimally invasive treatment modality, and dramatic responses to SRS have been observed. However, distant failure remains a significant issue. To address this problem, more effective chemotherapy treatments need to be developed.
Stereotactic Radiosurgery as the Sole Treatment
SRS is seldom used as the sole treatment for gross disease in ependymoma because most patients usually undergo external beam radiation therapy. There are scarce data in the literature on this approach. In a series from Indiana University, a 16-month-old child who had partial response to a subtotally resected cerebellopontine angle ependymoma received SRS as the sole treatment to delay external beam radiation therapy. The ependymoma progressed after 13 months, and salvage treatment with further chemotherapy and conformal radiation therapy was given.18
Although this approach appears to be sound, given the lack of adequate data from the literature documenting its efficacy and safety in this setting, SRS should typically not be offered as the sole treatment outside of a clinical trial setting. Table 20.1 summarizes the treatment outcomes of selected series.
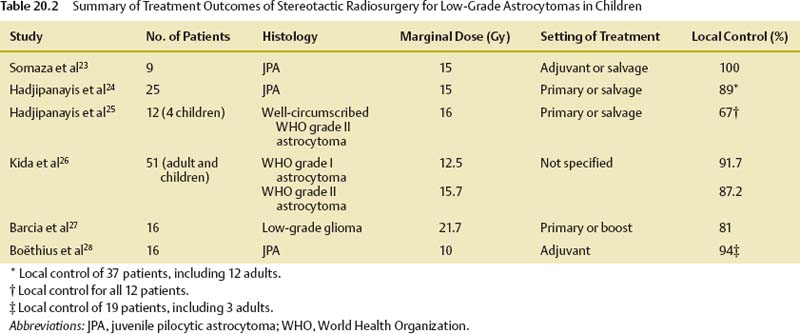
Low-Grade Astrocytomas
World Health Organization (WHO) grade I and II astrocytomas are collectively known as low-grade astrocytomas, which can occur in both adult and pediatric patients. Pilocytic astrocytomas (WHO grade I) represent the most indolent subtype of low-grade astrocytomas, with 10-year survival rates of over 90% in the pediatric population.22 Diffuse astrocytomas (WHO grade II) carry a slightly worse prognosis in the pediatric population, with 10-year survival rates of ~ 80%.22 The mainstay of treatment is maximal safe surgical resection, if achievable. If a complete resection is not achieved, chemotherapy is often given in young children to delay the need for immediate postoperative radiation therapy because of great concerns of collateral damage to the developing brain. However, if gross total resection or subtotal resection can be achieved, those children may be observed. SRS has been used in the treatment of low-grade astrocytoma in children in various settings, but the data in the literature are limited.
The data from the University of Pittsburgh represent one of the largest bodies of experience in the use of SRS in pediatric low-grade astrocytomas. In their early series, SRS was used as adjuvant treatment for growing and unresectable deep-seated pilocytic astrocytoma in nine children (two failed prior external beam radiation therapy). The mean prescribed dose was 15 Gy. With a median follow-up of 19 months, none of the patients treated developed progression (five had marked tumor shrinkage, and four had stable findings).23 None of the patients experienced any acute or late toxicity. In a subsequent study from the same institution, 37 patients (25 pediatric patients) with recurrent or unresectable pilocytic astrocytoma were treated with SRS. The prescribed dose was 15 Gy. Eighteen patients had brainstem tumors. Out of the 37 patients treated, 10 had complete response, 8 had partial response, 7 had stable findings, and 12 had delayed tumor progression on imaging studies.24 There was no treatment-related mortality. At a median follow-up of 28 months, the crude survival rate was 89%.24 The researchers also reported their treatment outcomes of SRS for WHO grade II fibrillary astrocytoma. A total of 12 patients (4 children) with progressive disease were treated with SRS to a median dose of 16 Gy. Four of the 12 patients had tumors in the brainstem. Tumor volumes ranged from 1.2 to 45.1 mL. At a median follow-up of 52 months, all patients were alive.25 Five patients had tumor shrinkage (one complete and four partial responses), three had stable findings, and four had delayed progression. The crude freedom from progression (FFP) rate was 67%.25
One of the largest series of SRS for low-grade astrocytoma was from Japan. A total of 51 adult and pediatric patients with low-grade astrocytomas were treated with Gamma Knife radiosurgery. Out of the 51 patients, 12 had WHO grade I astrocytomas around the optic pathway or hypothalamus. The remaining patients had WHO grade II astrocytomas. The mean age for patients with grade I astrocytomas was 9.8 years. The mean prescribed doses for grades I and II tumors were 12.5 and 15.7 Gy, respectively. The respective tumor diameters were 2.54 and 2.37 cm. With a median follow-up period of 27.6 months, the response rates for grades I and II astrocytomas were 50.0 and 46.2%, respectively.26 The corresponding tumor control rates were 91.7 and 87.2%, respectively.26 There was a significantly better response for patients 10 years of age or older with grade I tumors and those with a follow-up period of more than 24 months. Radiation-induced edema occurred in 18 (35.3%) patients, cyst formation or enlargement in 5 (9.8%), and transient tumor enlargement in 3 (5.9%).26
In a study from Spain, Barcia et al reported the treatment results of 16 patients with low-grade gliomas treated with SRS. Six patients had prior conventional radiation therapy. The mean dose given was 21.7 Gy. Complete response and partial response or stabilization were observed in 50 and 31% of the tumors treated, respectively.27 Three patients with tumors located in the brainstem died of tumor progression. Another study from Europe reported favorable treatment outcomes. Nineteen patients (16 children) from a Swedish institution were treated with SRS for pilocytic astrocytoma. The median tumor volume and the median prescribed dose were 2.2 mL and 10 Gy, respectively. Out of the 19 patients treated, 18 achieved tumor control at a median follow-up of 4.7 years.28 The tumor shrinkage rate was 85%. Increased contrast enhancement and edema attributable to SRS were observed in 25% of the patients.28 Table 20.2 summarizes the treatment outcomes of selected series.
As described by Larson et al, low-grade astrocytomas or gliomas are classified as category III targets, which are defined as targets that are early-responding tissue intermingled with normal brain parenchyma, which is late-responding tissue.6 It is more common to have normal brain parenchyma intermingled with the target tissue in WHO grade II than in WHO grade I astrocytomas. Based on the radiobiological characteristics described by Larson et al, low-grade astrocytomas are not regarded as ideal targets for SRS.6 Furthermore, as opposed to WHO grade I or pilocytic astrocytomas, WHO grade II tumors are usually less well demarcated from the normal brain parenchyma. Data in the literature regarding the use of SRS in children with newly diagnosed or recurrent low-grade astrocytomas are relatively limited. Despite the notion that low-grade astrocytomas are not ideal targets for SRS, data from various series have demonstrated good local control and acceptable toxicity profile. Figure 20.2 shows the treatment plan of a WHO grade I astrocytoma treated with SRS. It appears that SRS is safe and efficacious in the management of pediatric patients with low-grade astrocytoma and is especially useful in cases where there is a prior history of external beam radiation therapy and complete surgical resection is not possible. Currently, there are not enough data to support the routine use of SRS in children with newly diagnosed low-grade astrocytoma, and this is best tested in a clinical trial setting.
High-Grade Gliomas
High-grade gliomas constitute 15% of primary brain tumors in children and usually carry a dismal prognosis despite the improvement in treatments in the past 2 decades.2 They have a propensity to spread microscopically beyond the extent of tumor demonstrated on diagnostic imaging, but the main pattern of failure is still within 2 cm of the original tumor.29 In adults, prospective trials and studies have been done using hyperfractionation or addition of a boost with brachytherapy.30–32 Because of its favorable dosimetric characteristics, SRS has also been used as a boost therapy for dose escalation in adults with high-grade gliomas.33 SRS has also been used in patients with recurrent high-grade gliomas after prior external beam radiation therapy. However, data regarding the use of SRS for children with high-grade gliomas are very limited.
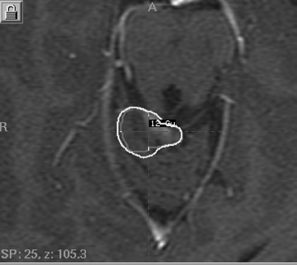
Fig. 20.2 A 5-year-old patient with WHO grade I (pilocytic) astrocytoma in the posterior fossa treated with Gamma Knife radiosurgery to a prescribed marginal dose of 12 Gy.
In a series from Harvard University, 18 patients with glioblastoma multiforme or anaplastic astrocytoma received SRS. Out of the 18 patients treated, only 4 of them, all of whom received SRS as boost therapy, survived, with progression after 50 to 119 months.16 The overall median survival time was 12 months after SRS. Other series showed similar dismal outcomes. In a study from the University of California, San Francisco, 11 children with high-grade gliomas were treated with SRS. Out of the 14 tumors treated, only 5 (36%) were controlled.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree