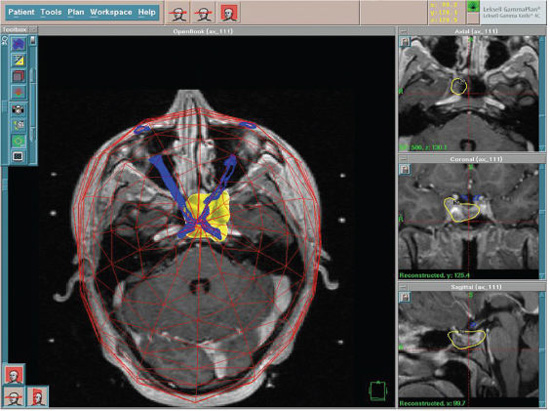
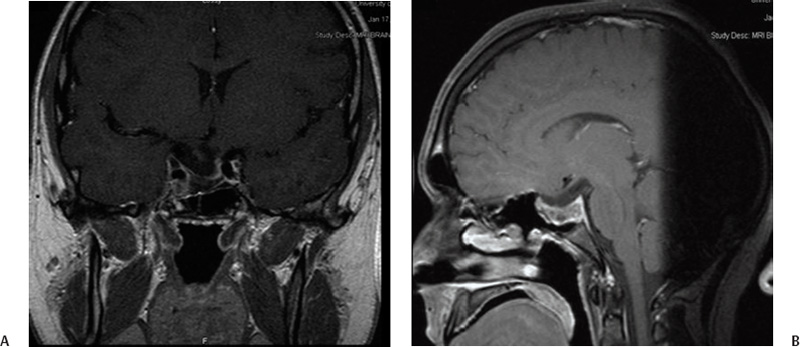
9 Pituitary adenomas represent 10 to 20% of all primary brain tumors.1,2 Lesions are broadly classified into two groups, depending on the presence or absence of secreted pituitary hormones. Tumors that secrete an excessive amount of a hormone are associated with various clinical syndromes. The most common secretory adenoma is the prolactinoma, which results in amenorrhea–galactorrhea syndrome and infertility in women and impotence and infertility in men. Growth hormone–secreting pituitary adenomas cause acromegaly in adults and gigantism in children, and tumors secreting adrenocorticotropic hormone (ACTH) cause Cushing’s syndrome. Nonsecretory tumors, which do not secrete biologically active pituitary hormones, account for ~30% of all pituitary adenomas. Adenomas in this group enlarge progressively in the pituitary fossa and often extend beyond the sella turcica.3 Tumor enlargement may cause local effects, including compression of the optic apparatus and visual field dysfunction. Patients with larger tumors might also have headache from elevated intracranial pressure and hypopituitarism resulting from compression of the normal pituitary gland. All patients suspected of harboring a pituitary tumor should undergo a complete neurologic, ophthalmologic, endocrinologic, and radiologic workup. This includes formal visual field and acuity testing and a dilated funduscopic examination.2,4 Each facet of the hypothalamic-pituitary-end organ axis should be assessed. Mild elevations in serum prolactin commonly result from a stalk effect, and levels > 200 ng/mL suggest a prolactin-secreting adenoma. Thyroid function is evaluated by measuring free thyroxine and thyroid-stimulating hormone (TSH). Adrenal function is assessed by a morning serum cortisol and ACTH level. In cases of suspected Cushing’s syndrome, a 24-hour urinary free cortisol (UFC) test and a dexamethasone suppression test are performed. Serum growth hormone and insulin like growth factor-1 (IGF-1) levels are measured to evaluate for acromegaly.5 Oral glucose tolerance with GH measurement is the definitive test in cases of suspected growth hormone–secreting tumors.6 In children, a radiograph should be obtained to assess bone age in relation to chronological age. Radiologic evaluation is achieved with thin-slice pre- and postcontrast magnetic resonance (MR) images of the sellar region. Computed tomography (CT) images may be useful to assess the degree of sinus aeration, particularly in younger patients who often have an underpneumatized sphenoid sinus.7,8 If a patient has a neurologic deficit attributable to an adenoma, surgery is the initial treatment of choice for all tumors except a prolactinoma. Transsphenoidal surgery (endoscopic or microscopic) allows for the most rapid relief of mass effect and reduction in excessive hormone levels in patients with Cushing disease and acromegaly.1,4,9–11 This approach is associated with a low rate of complications in the hands of an experienced neurosurgeon.12 Radiation therapy was originally the adjuvant treatment of choice for recurrent or residual pituitary adenomas, with reports dating to the early 20th century. However, conventional radiotherapy had several limitations. The time to hormonal normalization was slow, and the rate of postprocedural hypopituitarism was high. Side effects of conventional radiation treatment included cognitive difficulty, neuropsychological deficits, visual dysfunction from optic neuropathy, stroke, and damage to other cranial nerves. The advantage of stereotactic radiosurgery is that it delivers a high and tightly focused dose of radiation to a tumor in a single session. This single-session approach has a clear radiobiological advantage in late-responding tumors such as pituitary adenomas. With the aid of neuroimaging guidance, a steep radiation fall-off, and shielding achieved through blocking patterns, the surrounding tissues are largely spared from the harmful effects of radiation.13 This is particularly important in pituitary adenomas, as tumors may be located within millimeters of the cavernous sinus and optic apparatus. Shortly after radiosurgery’s development, Lars Leksell treated patients with pituitary tumors using the Gamma Knife (Elekta Instruments AB, Stockholm, Sweden); original localization was achieved through use of plain radiographs of the sella.14 Since that time, stereotactic radiosurgery with the Gamma Knife or modified linear accelerators (linacs) has been used in thousands of pituitary adenoma patients to control adenoma growth and normalize hormone overproduction.15–17 At the same time, great attention and effort in the field of stereotactic radiosurgery have been placed on the preservation of surrounding neuronal, vascular, and hormonal structures. Several radiosurgical treatment options exist for the pituitary, each with potential advantages and limitations. Proton beam radiosurgery was one of the earliest modalities used for the treatment of pituitary tumors.18,19 This technique takes advantage of the superior dose distribution of protons versus photons, due to the peak in the energy distribution of protons (“Bragg peak”) before they come to rest at the treatment depth.13 This gives the theoretical advantage of producing very little lateral disruption. Proton beam therapy can be used in the treatment of deep-seated lesions in the sella or near the cavernous sinus. Early studies on proton beam radiosurgery were complicated by high rates of hypopituitarism, a factor likely related to the nascent treatment planning of the time.18 Reports on modern proton beam therapy have a lower rate of hypopituitarism. The major limitation of the technique is that proton beam (cyclotron) facilities are available only at a very limited number of centers worldwide.20 In linac-based radiosurgery, multiple radiation arcs are used to crossfire photon beams at a target defined in stereotactic space.21–23 Most of the current systems use nondynamic techniques in which the patient couch is set at an angle, and the arc is moved around its radius to deliver radiation that enters the skull through many different points. Numerous techniques have been developed to enhance conformity of dose planning and delivery using linac-based systems. These include beam shaping and intensity modulation.24–26 Newer developments include the introduction of jaws and noncircular and mini- and microleaf collimators.27 The conformal beam can be delivered with the micromulti-leaf collimator or conformal blocks. Gamma Knife radiosurgery usually involves multiple isocenters of different beam diameter to achieve a dose plan that conforms to the irregular three-dimensional volumes of most mass lesions. The total number of isocenters may vary depending on the size, shape, and number of lesions. The most recent version of the Gamma Knife, the Perfexion, combines advances in dose planning, beam collimation, and robotic engineering, and it eliminates the need to set coordinates manually for each isocenter. No published comparisons of Gamma Knife multiple isocenter techniques, linac or proton beam, have shown a clear advantage of one technique over another with regard to sparing normal tissue, achieving endocrine remission, or reducing complications.28,29 As with other neurosurgical procedures, the outcome may depend less on the radiosurgical device and more on the experience of the radiosurgical team. To paraphrase neurosurgeon Charles Wilson, it is not just the horse but also the jockey that makes the difference in winning a race. In 2000 Landolt et al reported a significantly lower hormone normalization rate in patients with acromegaly who were receiving antisecretory medications at the time of radiosurgery.30 The precise mechanism by which antisecretory medications lower hormonal normalization rates is unknown, but it is thought to be related to changes in cell cycling caused by these drugs, potentially decreasing tumor cell radiosensi-tivity.30,31 Since 2000, several other groups (e.g., Mayo, Marseille, and the University of Virginia) have documented a counterproductive effect of antisecretory medications on the rate of hormonal normalization following radiosurgery for patients with acromegaly and prolactinomas.30,32,33 Although the optimal time period to hold antisecretory medications is unclear, published studies have indicated that long-acting somatostatin analogues should probably be discontinued 8 weeks before treatment and resumed 6 to 8 weeks after radiosurgery if the benefit of radiosurgery is to be optimized.30–34 Based on these reports, many centers have advocated the cessation of antisecretory medications. However, as is true for much of neurosurgical practice, class I evidence is still unavailable to support this treatment approach. Ultimately, the radiosurgical team must weigh the potential risk and benefits of altering antisecretory medication administration. In the absence of antisecretory medication control, a tumor may rarely enlarge, thereby risking adjacent structures (e.g., the optic apparatus), necessitating a lower prescription dose, and making effective treatment more difficult. On the day of the treatment, the stereotactic head frame is applied in the standard fashion.35,36 We find that patients are more comfortable when the frame is placed in the operating room using intravenous sedation with an anesthesiologist or nurse anesthetist in attendance. Prior to frame placement, the scalp is prepared with alcohol, and the areas of the pin placements are infiltrated with a long-acting local anesthetic. It is generally useful to use the angle of the optic apparatus as the axis of the frame. This angle approximates a line joining the lateral canthus and the top of the pinna, and it makes identification of the optic nerves, chiasm, and tracts easier by having an image that demonstrates the entire optic apparatus in a single MRI slice.37 Fig. 9.1 (A) Coronal and (B) sagittal postcontrast magnetic resonance images in an 18-year-old right-handed male with Cushing’s disease. The patient presented with rapid visual loss, which improved after transsphenoidal resection. However, he had residual tumor encasing the cavernous sinus and carotid artery, as well as biochemical evidence of persistent Cushing’s disease. Regardless of the radiosurgical modality used, stereotactic radiosurgery requires clear and accurate imaging of that target. Advances in radiographic imaging over the past 20 years have increased the efficacy and safety of radiosurgical treatment of pituitary lesions. Modern imaging consists of an MRI sequence consisting of thin-slice (e.g., 1 mm), post-contrast volume acquisition to define the tumor within the sellar region. In patients with previous surgery, fat suppression techniques can prove useful for differentiating tumor from surgical fat grafts (Fig. 9.1). In the pre-MRI era, CT was used routinely, but it is now used only when a patient cannot tolerate an MRI. Positron emission tomography (PET) imaging may also be of value for detecting hypersecretory adenomas; such images can be overlaid on stereotactic tomographic images (e.g., MRI or CT).38 After the acquisition of images with the stereotactic frame, multiple isocenter dose planning is performed to enclose the borders of the tumor within the prescription isodose line.39,40 The 50% isodose is the most common prescription isodose line for Gamma Knife radiosurgery because this line is generally where the slope of the radiation fall-off is the steepest (Fig. 9.2).41 However, one should analyze the dose gradient and determine the optimal fall-off isodose, which generally varies between 40 and 60%.42 Beam-blocking plug patterns are often used to shift the prescription isodose curves away from the optic apparatus because of the radiosensitivity of this structure. These “plugging patterns” may be generated manually or by using planning software and are then implemented by replacing collimators in the helmet with solid “plugs.” The planning software currently in use at most Gamma Knife centers (Leksell GammaPlan versions 5.33–4C; Elekta Instruments, Inc., Norcross, GA) supports two general strategies for plugging: a “merged” plugging strategy and a “custom” plugging strategy. The merged plugging strategy uses the software to create plug patterns for each helmet, which are placed at the union of software-computed patterns for each isocenter. These lowest common denominator patterns for each helmet size are then applied to every isocenter of that size. The advantage of this feature is that much of the work required to define plugging patterns and assign them to isocenters is automated by the software, requiring no plugging pattern changes during treatment other than when changing collimator helmets. The custom plugging strategy involves generating combinations of plugging patterns that are manually applied to one or more isocenters in a dose plan. Isocenters that require similar shielding effects are given similar or identical plugging patterns.43 With the Gamma Knife Perfexion, plugging can be accomplished by closing off any one or a combination of eight sectors that comprise the 192-source collimator. Sectors are closed in an automated fashion. The need to use plug patterns can be reduced by adjusting the gamma angle so that the anteroposterior axis of the peripheral isodose curves is parallel to the optic apparatus in the sagittal plane. If the frame is placed parallel to the course of the optic apparatus, then a gamma angle of 72 or 90 degrees may be used. This maneuver takes advantage of the extremely steep fall-off of radiation dorsal to the isocenter. Selection of the prescription dose is based partially on the integrated logistic formula as well as specific strategies for protecting the optic apparatus, controlling tumor growth, and establishing and maintaining normal endocrinologic function.44,45 Visual deterioration following radiosurgery is rare and can generally be avoided if the dose to the optic apparatus is restricted to < 8 Gy, although reports of 10 to 12 Gy have been described by some groups.46 Traditionally, a distance of 3 mm or more between the adenoma and the optic apparatus is desirable. The absolute distance between the optic apparatus is not the limiting factor, but rather defines how steeply the radiation gradient must be constructed so that a tolerable dose is delivered to the optic apparatus while still delivering an effective dose to the adenoma. If an acceptable gradient cannot be constructed, then alternative treatment should be considered. The Gamma Knife Perfexion may allow a distance of as little as 1 to 2 mm.17,47–50 Ultimately, the tolerable absolute dose permitted likely varies from patient to patient, and it is affected by factors such as previous damage to the optic apparatus by pituitary adenoma compression, ischemic changes, type and timing of previous interventions (e.g., fractionated radiation therapy and surgery), the patient’s age, and the presence or absence of other comorbidities.33,51 The majority of cranial nerves in the cavernous sinus appear to be more resistant to radiation effects than the optic nerve, but reports of cranial neuropathy, particularly after repeat radiosurgery, are well documented.51 Although the tolerable limit to the cavernous sinus nerves is unknown, authors have described effective radiosurgical doses of between 19 and 23 Gy to this region without major side effects.17,52–56 Injury to the cavernous segment of the carotid artery is rare after radiosurgery, with a few isolated case reports.57 In cases where the tumor appears to extend into the cavernous sinus, blocking patterns can be used to deliver radiation while shielding vital structures.
Stereotactic Radiosurgery for Pituitary Adenomas
Jay Jagannathan, David J. Schlesinger, Rod J. Oskouian Jr., Nader Pouratian, John A. Jane Jr., Edward R. Laws, Mary Lee Vance, and Jason P. Sheehan
 Patient Evaluation
Patient Evaluation
 History and Evolution of Stereotactic Radiosurgery for Pituitary Adenomas
History and Evolution of Stereotactic Radiosurgery for Pituitary Adenomas
 Radiosurgical Treatment Options for Pituitary Tumors
Radiosurgical Treatment Options for Pituitary Tumors
 Radiosurgical Planning and Technique
Radiosurgical Planning and Technique
Cessation of Suppressive Medications
Frame Placement
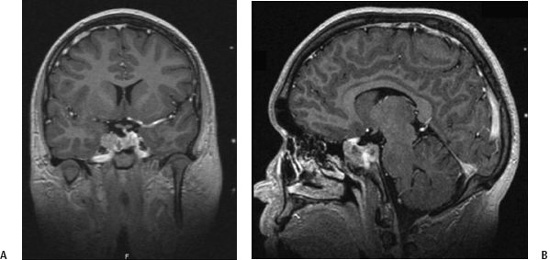
Radiographic Imaging
Treatment Planning
Plugging Strategies
Neuroanatomical Considerations: Radiation Effects on the Optic Apparatus, Cavernous Sinus, and Pituitary Gland
Stereotactic Radiosurgery for Pituitary Adenomas