Stomach and Duodenum
William E. Brant
Imaging Methods
As endoscopy has become more commonplace, the utilization of fluoroscopy to study the upper gastrointestinal (UGI) tract has continued to diminish (1). CT competes with endoscopic US to evaluate the extraluminal component of disease (2,3). Nonetheless, a high-quality UGI series provides excellent evaluation of the stomach and duodenum and remains part of the radiologic armamentarium (4). To attain a high sensitivity for the examination and to avoid missing significant pathology, multiple techniques must be used for the UGI series. Single-contrast technique involves filling and distending the stomach and duodenum with barium suspension followed by compression procedures to demonstrate abnormalities of the distal stomach and duodenum. Mucosal relief technique entails using small amounts of barium to coat the mucosa without distending the bowel to demonstrate abnormalities such as varices. Double-contrast technique, using high-density barium suspensions to coat the mucosa and ingestible effervescent granules to distend the stomach and duodenum, is optimal for the demonstration of subtle features of the mucosal surface (4). As with any radiographic examination, attention to detail and tailoring the examination to address the clinical problem is essential in producing optimal results.
CT, with the use of air-contrast distention techniques, is a valuable adjunct to barium studies and endoscopy to document the abnormalities of the wall of the stomach and duodenum and to determine the extent of extraluminal disease (2,3,5). Optimal distension of the stomach and duodenum is mandatory for accurate CT interpretation. Gastric and duodenal distension may be attained by filling the organs with water, positive contrast agents, or by ingesting effervescent granules to cause gaseous distension. The patient is positioned to optimize the distension of the GI tract portion of greatest interest. MR and US play increasing roles in the evaluation of the luminal GI tract (6).
Anatomy
The GI tract is essentially a hollow tube consisting of four concentric layers of tissue. The innermost layer exposed to the lumen is the mucosa. The mucosa consists of epithelium supported by loose connective tissue of the lamina propria and a thin band of smooth muscle called the muscularis mucosae. The submucosa provides connective tissue support for the mucosa. The submucosa contains the primary vascular and lymphatic channels, lymphoid follicles, and autonomic nerve plexuses. The major muscular structure of the bowel wall is the muscularis propria, comprised of inner circular and outer longitudinal layers. The serosa or adventitia is the outer covering of the bowel. Lymphoid tissue in the GI tract is located in the mucosa (epithelium and lamina propria), the submucosa, and the mesenteric lymph nodes. As the major component of the mucosa-associated lymphoid tissue (MALT), lymphoid tissue plays a major role in host immune defenses and is a site of significant disease (7).
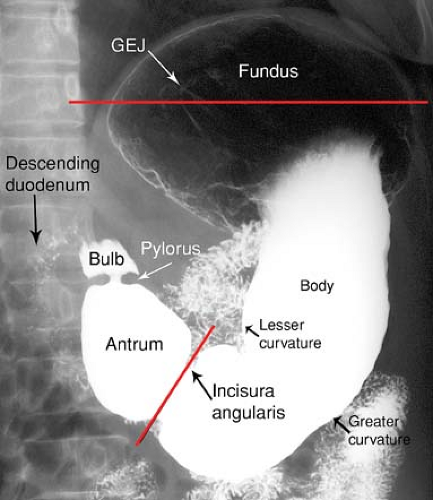
The appearance and position of the stomach and duodenum vary considerably from one individual to another. The terms used to describe the anatomic divisions of the stomach and duodenum are illustrated in Figure 29.1 (4). Cardia refers to the region of the gastroesophageal junction (GEJ). The fundus is that portion of the stomach above the level of the GEJ. The body of the stomach is the central two-thirds portion from the cardia to the incisura angularis. The incisura angularis is the acute angle formed on the lesser curvature that marks the boundary between the body and the antrum. The parietal cells, which produce hydrochloric acid, and the chief cells, which produce pepsin precursors, are located in the fundus and the body. The antrum is the distal one-third of the stomach and contains gastrin-producing cells but no acid-secreting cells.
The pylorus is the junction of the stomach with the duodenum, and the pyloric canal is the channel through the pylorus. The duodenal bulb, or cap, is the pyramidal first portion of the duodenum. The gallbladder frequently makes a prominent impression on the top of the bulb. The duodenum bulb, like the stomach, is covered on all surfaces by visceral peritoneum. The remainder of the duodenum is retroperitoneal and within the anterior pararenal compartment.
The second or descending portion of the duodenum is lateral to the head of the pancreas. The common bile duct and pancreatic duct pierce the medial aspect of the descending duodenum at the ampulla of Vater. The third or horizontal portion of the duodenum passes to the left between the superior mesenteric vessels and the inferior vena cava and aorta.
The fourth or the ascending portion of the duodenum ascends on the left side of the aorta to the level of L-2 and the ligament of Treitz, where it turns abruptly ventrally to form the duodenal–jejunal flexure.
The fourth or the ascending portion of the duodenum ascends on the left side of the aorta to the level of L-2 and the ligament of Treitz, where it turns abruptly ventrally to form the duodenal–jejunal flexure.
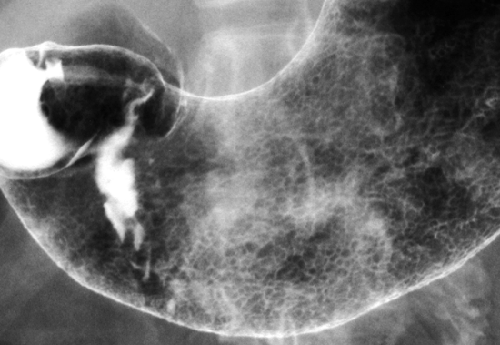
The term areae gastricae refers to the detailed pattern of the gastric mucosa as demonstrated by double-contrast technique (Fig. 29.2). Normal areae gastricae varies from a fine reticular pattern to a course nodular pattern. The hallmark of normal is the regularity of the pattern in all areas in which it is visualized. The term rugae refers to the gastric mucosal folds that produce distinct radiolucent ridges when the stomach is partially distended. Rugae are composed of mucosa, the lamina propria, the muscularis mucosae, and portions of the submucosa. Disease in any of these structures may cause thickening of the gastric folds. Rugal folds are most prominent in the fundus and proximal gastric body and are usually absent in the antrum.
The lesser curvature of the stomach is attached to the liver by the lesser omentum. The greater omentum attaches to the greater curvature of the stomach. The lesser sac is the intraperitoneal space posterior to the stomach and anterior to the pancreas.
On CT, the normal gastric wall when well distended in the antrum is 5 to 7 mm thick and in the body 2 to 3 mm thick. The wall of the normal duodenal is less than 3 mm thick. Both organs must be fully distended to accurately assess the wall thickness. A prominent pseudotumor, caused by inadequate distension, is often seen on CT near the GEJ.
Stomach
Helicobacter pylori Infection
H. pylori infection has been identified as the major cause of chronic gastritis, duodenitis, benign gastric and duodenal ulcers, gastric adenocarcinoma, and MALT lymphoma (4). H. pylori is a gram-negative spiral bacillus that colonizes the stomachs in as many as 80% of individuals in some populations. It will infect only gastric-like epithelium and is usually localized to the gastric antrum, living on the surface epithelial cells beneath the mucous coat. It survives in gastric acid by using a powerful urease enzyme to break down urea into ammonia and bicarbonate, creating a more alkaline environment for itself. The prevalence of infection increases with age (>50% of Americans older than 60 years) and is high in lower socioeconomic populations and in developing countries. Infection is chronic and causes a superficial gastritis, which is most commonly asymptomatic. Approximately 70% of peptic gastric ulcers, 95% of duodenal ulcers, and 50% of gastric adenocarcinoma are caused by this infection. Double-contrast technique demonstrates enlarged areae gastricae in 50% of patients with H. pylori infection. Diagnosis of H. pylori infection is made by serology, urease breath tests, and endoscopic biopsy. Treatment is usually a combination of two to four drugs including one or more antibiotics, H2 blockers to decrease acid secretion, and occasionally a bismuth compound. Cure rates of 90% are reported although antibiotic resistance is emerging. Although spontaneous elimination of infection is rare, treatment of asymptomatic infected individuals is not currently recommended.
Gastric Filling Defects/Mass Lesions
Gastric carcinoma is the third most common GI malignancy, following colon and pancreatic carcinoma. Most (95%) are adenocarcinomas; the remainder are diffuse anaplastic (signet-ring) carcinoma, squamous cell carcinoma, or rare cell types (5). Predisposing factors include smoking, pernicious anemia, atrophic gastritis, and gastrojejunostomy. H. pylori infection increases the risk of gastric carcinoma sixfold and is the cause of approximately half of gastric adenocarcinoma cases. The peak age is from 50 to 70 years, with males predominating
2:1. The incidence of gastric carcinoma is as much as five times higher in Japan, Finland, Chile, and Iceland than in the United States. Mortality is high with a 5-year survival rate of 10% to 20%.
2:1. The incidence of gastric carcinoma is as much as five times higher in Japan, Finland, Chile, and Iceland than in the United States. Mortality is high with a 5-year survival rate of 10% to 20%.
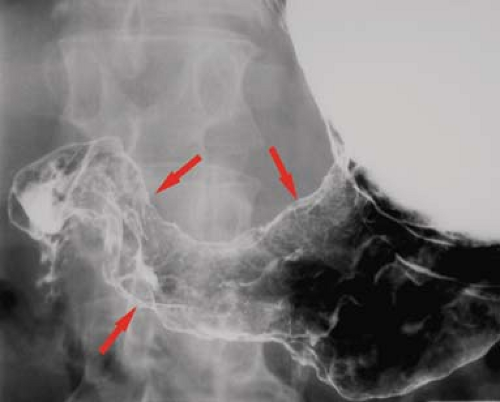
 Figure 29.3. Polypoid Gastric Carcinoma. Single-contrast technique upper GI series reveals a lobulated filling defect (arrows) in the antrum of the stomach. |
The tumor assumes four common morphologic growth patterns. One-third are polypoid masses that present as filling defects within the gastric lumen (Fig. 29.3). Many of these are broad based and papillary in configuration. Another one-third are ulcerative masses presenting as malignant gastric ulcers. The remainder are infiltrating tumors, focal plaque-like lesions with central ulcer, or diffusely infiltrating (15%) with poorly differentiated carcinomatous cells producing bizarre thickened folds and thickened rigid stomach wall, the so-called scirrhous carcinomas (Figs. 29.4, 29.5). The terms “linitis plastica” and “water-bottle stomach” may be applied to describe the resulting stiff narrowed stomach. Additional causes of narrowed stomach are listed in Table 29.1.
The tumor spreads by direct invasion through the gastric wall to involve perigastric fat and adjacent organs, or it may seed the peritoneal cavity. Lymphatic spread is to the regional lymph nodes including perigastric nodes along the lesser curvature, celiac axis, and hepatoduodenal, retropancreatic, mesenteric, and para-aortic nodes (8). Hematogenous metastases involve the liver, adrenal glands, ovaries, and, rarely, bone and lung. Intraperitoneal seeding presents as carcinomatosis or Krukenberg ovarian tumors. PET-CT is most effective in the demonstration of metastatic lymph nodes and distant spread of tumor (8).
Early gastric cancers appear on barium studies as (1) gastric polyps with risk of malignancy increased for lesions larger than 1 cm, (2) superficial plaque-like lesions or nodular mucosa, and (3) shallow, irregular ulcers with nodular adjacent mucosa. These lesions are most sensitively detected on double-contrast studies.
CT and MR are used to determine the extent of tumor to facilitate preoperative planning (Fig. 29.6) (9). Transmural
extension, intraperitoneal spread, or distant metastases limit the treatment to palliative surgery or chemotherapy. Findings include (1) focal, often irregular, wall thickening (>1 cm); (2) diffuse wall thickening due to tumor infiltration (linitis plastica) (contrast enhancement is common); (3) intraluminal soft tissue mass; (4) bulky mass with ulceration; (5) rare, large, exophytic tumor resembling leiomyosarcoma; (6) extension of tumor into perigastric fat; (7) regional lymphadenopathy; and (8) metastases in the liver, adrenal, and peritoneal cavity. Mucinous adenocarcinomas frequently contain stippled calcifications. Findings used to differentiate malignant gastric neoplasms are listed in Table 29.2.
extension, intraperitoneal spread, or distant metastases limit the treatment to palliative surgery or chemotherapy. Findings include (1) focal, often irregular, wall thickening (>1 cm); (2) diffuse wall thickening due to tumor infiltration (linitis plastica) (contrast enhancement is common); (3) intraluminal soft tissue mass; (4) bulky mass with ulceration; (5) rare, large, exophytic tumor resembling leiomyosarcoma; (6) extension of tumor into perigastric fat; (7) regional lymphadenopathy; and (8) metastases in the liver, adrenal, and peritoneal cavity. Mucinous adenocarcinomas frequently contain stippled calcifications. Findings used to differentiate malignant gastric neoplasms are listed in Table 29.2.
Table 29.1 Narrowed Stomach | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Lymphoma accounts for 2% of gastric neoplasms (10). The stomach is the most common site of involvement of primary GI lymphoma, accounting for approximately 50% of cases. Most (80%) gastric lymphoma is non-Hodgkin, B-cell type (9). Chronic infection of the gastric epithelium with H. pylori is associated with the risk of developing MALT gastric lymphomas, which are more indolent and have a better prognosis than B-cell lymphomas (7). Because lymphoma remains confined to the bowel wall for prolonged periods of time, it has a better prognosis than carcinoma with a 5-year survival rate of 62% to 90%. Lymphoma demonstrates four morphologic patterns: polypoid solitary mass, ulcerative mass, multiple submucosal nodules (Fig. 29.7), and diffuse infiltration (Fig. 29.8). UGI findings include the following: (1) polypoid lesions, (2) irregular ulcers with nodular thickened folds, (3) bulky tumors with large cavities, (4) multiple submucosal nodules that commonly ulcerate and create a target or “bull’s-eye” appearance, (5) diffuse but pliable wall and fold thickening, and (6) rarely, linitis plastica appearance of diffuse, stiff narrowing (Fig. 29.8) (7). Multiplicity of lesions favors MALT lymphoma as the diagnosis.
CT is the primary imaging modality used to stage lymphoma. CT findings that are helpful in differentiating gastric lymphoma from carcinoma include (1) more marked thickening of the wall (may exceed 3 cm) (Fig. 29.9), (2) involvement of additional areas of the GI tract (trans-pyloric spread of lymphoma to the duodenum in 30%), (3) absence of invasion of the perigastric fat, (4) absence of luminal narrowing and obstruction despite extensive involvement, and (5) more widespread and bulkier adenopathy (10).
GI stromal tumors (GISTs) are the most common mesenchymal tumors to arise from the GI tract (11,12). Most, but not all, tumors previously classified as leiomyomas,
leiomyosarcomas, and leiomyoblastomas are now classified as GISTs. Approximately 60% to 70% of GISTs arise in the stomach, and 10% to 30% of these are malignant. True leiomyomas and leiomyosarcomas are very rare in the stomach.
leiomyosarcomas, and leiomyoblastomas are now classified as GISTs. Approximately 60% to 70% of GISTs arise in the stomach, and 10% to 30% of these are malignant. True leiomyomas and leiomyosarcomas are very rare in the stomach.
Table 29.2 Gastric Malignancies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
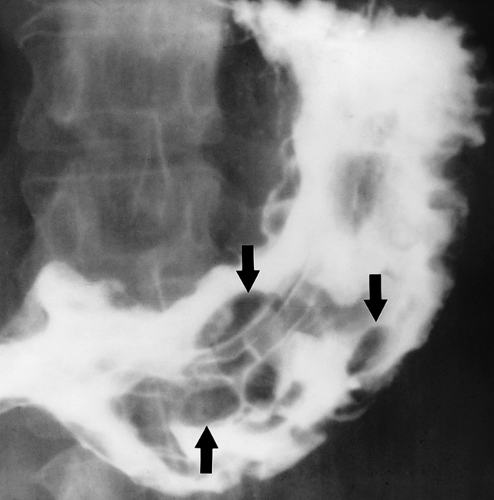 Figure 29.7. Gastric Lymphoma—Multinodular. Upper GI series shows multiple smoothly marginated polypoid nodules of varying size and shape in the stomach. Multiple polypoid nodules may also be seen with gastric carcinoma.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|