The breadth of tumors that can arise in the supratentorial brain in children is extensive. With the exception of those that result in seizures and the highly malignant histologies, supratentorial tumors may come to medical attention later compared with infratentorial tumors, as they are less commonly associated with ventricular obstruction. This article presents an overview of the neuroimaging characteristics of these entities, with particular attention to relevant features that may aid in narrowing the differential diagnosis, including correlation with demographics and clinical presentation.
Key points
- •
Anaplastic transformation of diffuse astrocytomas is a much less common event in children compared with adults.
- •
Both subependymal nodules and subependymal giant cell tumors can show contrast enhancement.
- •
Contrast enhancement and calcifications in pediatric oligodendrogliomas are less common than in adults.
- •
Almost all gangliogliomas are low grade and present as cystic and solid mass lesions, most frequently arising in the temporal lobes.
- •
Up to one-third of dysembryoplastic neuroepithelial tumors may show contrast enhancement that may be nodular or ring-like.
Introduction
Brain and central nervous system (CNS) tumors continue to represent a significant source of morbidity and mortality in the pediatric population. They are the most common solid tumors in children between 0 to 14 years of age, and their incidence is highest during the first year of life. These tumors account for the most cancer-related deaths in the 0 to 14 age group according to the Central Brain Tumor Registry of the United States (CBTRUS). Overall, most brain tumors in children are gliomas, with roughly half of them consisting of pilocytic astrocytomas or other low-grade neoplasms, followed by embryonal tumors. Approximately 21% of all gliomas have a high-grade histology and are associated with an aggressive clinical behavior and a dismal prognosis. When brain stem tumors are excluded, high-grade gliomas are most commonly supratentorial, occurring in the cerebral hemispheres, followed by central gray matter structures.
Fifteen percent of all CNS neoplasms are embryonal tumors, a heterogeneous group of lesions that arise from undifferentiated small round cells, tend to occur in small children, and are associated with a poor prognosis and a tendency to disseminate throughout the neuraxis. With the exception of medulloblastomas, embryonal tumors are predominantly supratentorial. Finally, although neuronal and mixed neuronal-glial tumors are not as common, accounting for less than 5% of all neoplasms, they may nonetheless lead to significant morbidity in many patients due to intractable seizures. Many of these lesions share similar clinical and imaging presentations making their prospective diagnosis challenging. This article reviews the neuroimaging characteristics of these entities with particular attention to relevant features that may aid in narrowing the differential diagnosis, including demographics and clinical presentation.
Introduction
Brain and central nervous system (CNS) tumors continue to represent a significant source of morbidity and mortality in the pediatric population. They are the most common solid tumors in children between 0 to 14 years of age, and their incidence is highest during the first year of life. These tumors account for the most cancer-related deaths in the 0 to 14 age group according to the Central Brain Tumor Registry of the United States (CBTRUS). Overall, most brain tumors in children are gliomas, with roughly half of them consisting of pilocytic astrocytomas or other low-grade neoplasms, followed by embryonal tumors. Approximately 21% of all gliomas have a high-grade histology and are associated with an aggressive clinical behavior and a dismal prognosis. When brain stem tumors are excluded, high-grade gliomas are most commonly supratentorial, occurring in the cerebral hemispheres, followed by central gray matter structures.
Fifteen percent of all CNS neoplasms are embryonal tumors, a heterogeneous group of lesions that arise from undifferentiated small round cells, tend to occur in small children, and are associated with a poor prognosis and a tendency to disseminate throughout the neuraxis. With the exception of medulloblastomas, embryonal tumors are predominantly supratentorial. Finally, although neuronal and mixed neuronal-glial tumors are not as common, accounting for less than 5% of all neoplasms, they may nonetheless lead to significant morbidity in many patients due to intractable seizures. Many of these lesions share similar clinical and imaging presentations making their prospective diagnosis challenging. This article reviews the neuroimaging characteristics of these entities with particular attention to relevant features that may aid in narrowing the differential diagnosis, including demographics and clinical presentation.
Glial cell tumors
Low-Grade Gliomas
World Health Organization (WHO) grade 1 and 2 gliomas roughly account for 60% of all gliomas in children. They are considered benign and usually follow a relatively indolent course with an overall 10-year survival exceeding 80%. However, these tumors may be associated with significant morbidity and even mortality with increasingly recognized leptomeningeal spread in pilocytic astrocytomas and malignant transformation in diffuse astrocytomas, although the latter is less commonly seen than in adults.
Pilocytic astrocytoma
Pilocytic astrocytomas account for one-third of all gliomas in children from 0 to 14 years of age and constitute the most common primary brain tumor in this population. Their incidence is relatively evenly distributed across this age group after the first year of life. They are histologically benign (WHO grade I) and demonstrate slow growth over time. Pilocytic astrocytomas have an excellent prognosis, with survival rates as high as 95% at 10 years. They most commonly occur in the cerebellar hemispheres (about two-thirds of lesions in pediatric patients), followed by optic chiasm and nerves and hypothalamus, but they can rarely develop in the cerebral hemispheres (particularly in older children and adults, accounting for half of all tumors in the latter group). Most pilocytic astrocytomas are sporadic, but there is a higher incidence in neurofibromatosis type 1, where they occur in up to 20% of patients. Notably, approximately one-third of patients with an optic pathway glioma (the majority of which are pilocytic) have neurofibromatosis type 1. Most pilocytic astrocytomas harbor a BRAF-KIAA1549 fusion gene mutation, which may be associated with improved clinical outcomes.
Nearly all pilocytic astrocytomas are well circumscribed on imaging, and approximately two-thirds of those in the cerebellum present with the characteristic appearance of a cystic mass with an avidly enhancing mural nodule. The cyst wall rarely enhances. In the cerebral hemispheres, the frequency of this appearance is unknown but appears to be less common than in the posterior fossa. A prior study has shown that approximately 36% of all cerebral astrocytomas present with cystic changes ( Fig. 1 ). Pilocytic astrocytomas may also appear as solid enhancing masses ( Fig. 2 ). On occasion they may demonstrate an infiltrating pattern in the surrounding tissue and even leptomeningeal spread, which renders their distinction from high-grade tumors challenging ( Fig. 3 ). An additional characteristic feature is the lack of significant vasogenic edema in the surrounding parenchyma. When edema does occur, it tends to be limited in relation to the size of the tumor.
Pilocytic astrocytomas are exceptional tumors in that they commonly show avid enhancement despite their benign and relatively indolent biology. They can also show an aggressive profile on magnetic resonance spectroscopy (MRS) that may be mistaken for a high-grade tumor, with increased choline, decreased N-acetylaspartate, and a lipid-lactate peak. However, recent data suggest that pilocytic astrocytomas have higher lipid–lactate/creatine ratios compared with high-grade tumors. The enhancing components of pilocytic astrocytomas tend to have low perfusion with decreased relative cerebral blood volumes (rCBV), although nodules with increased perfusion may at times be encountered. They also show significantly higher apparent diffusion coefficient (ADC) values compared with high-grade tumors by virtue of their low cellularity. Malignant transformation of pilocytic astrocytomas has been described but is an unusually rare event. Some studies suggest that this may be much more common in adults.
Diffuse astrocytomas
Diffuse astrocytomas are low-grade tumors (WHO grade II) that are several times less common in children than pilocytic astrocytomas. They can occur anywhere in the CNS, but one-third arise in the frontal or parietal lobes, which represent the most common location. On MR imaging, they have relatively ill-defined margins but are homogeneously hypointense on T1- and hyperintense on T2-weighted sequences, without contrast enhancement or restricted diffusion ( Fig. 4 ). Interestingly, while in adults most diffuse low-grade astrocytomas eventually undergo anaplastic transformation, progression to a higher-grade tumor is a rare event in children and accounts for only about 10% of cases. Diffuse astrocytomas do not show significantly increased rCBV and may show elevated myoinositol on MRS.
High-Grade Gliomas
High-grade gliomas are significantly less common in children than in adults, yet they constitute 11% of all CNS neoplasms in the pediatric population, with an estimated incidence of 0.59 per 100,000 person–years. Supratentorial high-grade gliomas comprise one-third of all pediatric high-grade gliomas and occur most commonly in adolescents. They may be related to prior radiation exposure or occur in the setting of rare syndromes such as Li Fraumeni. Most-high grade gliomas in children are purely astrocytic and classified as either anaplastic astrocytomas (WHO grade III) or glioblastomas (WHO grade IV), with other mixed or nonastrocytic types being rare in this population. Notably, evidence shows that pediatric high-grade gliomas are genetically and molecularly distinct from their adult counterparts.
Anaplastic astrocytoma
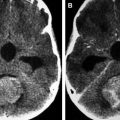
Anaplastic astrocytomas represent close to 2% of all CNS tumors in children. They are rapidly growing and infiltrative lesions with poorly circumscribed margins and are most commonly found in the cerebral hemispheres (particularly frontal and temporal lobes), although they can occur in the deep midline structures, brain stem, or cerebellum. They do not show significant contrast enhancement, hemorrhage, or necrosis, features that are associated with glioblastomas ( Fig. 5 ). ADC values of anaplastic astrocytomas are lower than those of pilocytic or diffuse astrocytomas, and they also show increased rCBV compared with lower-grade histologies ( Fig. 6 ). On MRS, anaplastic astrocytomas show increased choline and decreased N-acetylaspartate, with decreased myoinositol compared with the spectra of lower-grade gliomas.
Glioblastoma
Glioblastomas are rare in children, in whom they constitute about 3% of primary brain tumors. Survival is poor but better than that of adult glioblastomas. Most glioblastomas occur in the frontotemporal region but can also affect other lobes or the deep gray structures. The imaging hallmark of glioblastoma is that of heterogeneous enhancement with necrosis and marked peritumoral edema ( Fig. 7 ). The solid components show restricted diffusion with low ADC values as well as increased MR imaging perfusion and permeability parameters (such as rCBV and K trans ), which may be helpful in differentiating them from low-grade gliomas or for evaluation of tumor recurrence versus treatment response. On MRS, in addition to decreased myoinositol, decreased N-acetylaspartate, and elevated choline, glioblastomas typically have elevated lactate due to anaerobic metabolism and elevated lipids due to the presence of necrosis. Note that most of these studies have been performed in adults due to the rarity of these tumors in children.
Subependymal giant cell tumor
Subependymal giant cell tumors (SGCTs) are slow-growing neoplasms characterized as WHO grade I. They show mixed glioneuronal lineage and are not pure astrocytomas. SGCTs are most commonly seen in children and adolescents with tuberous sclerosis complex (TSC), in whom they constitute the most common CNS neoplasm (5%–20% of patients). It is unusual to develop an SGCT after age 21 years if not already present, although tumors that have been diagnosed in childhood can become symptomatic later. Several cases of solitary SGCTs have been described in patients without other manifestations of TSC. However, genetic testing in some isolated SGCTs has demonstrated mutations in the TSC-1 and TSC-2 genes, suggesting that at least some of these tumors may represent a forme fruste of TSC in patients without other clinical manifestations of the disease. They are supratentorial and virtually always located in a lateral ventricle near the foramen of Monro, although they may rarely occur in other locations. SGCTs appear to arise from neoplastic transformation of existing subependymal nodules, but the reason why some nodules grow and others do not is not clear. Enhancement is variable but usually avid and heterogeneous ( Fig. 8 ). However, in and of itself, contrast enhancement is not sufficient for diagnosis, as many subependymal nodules have also been shown to enhance. Both subependymal nodules and SGCTs can calcify and hemorrhage. From a clinical standpoint, the most important factor in the evaluation of a subependymal nodule or SGCT is the development of intracranial hypertension with new papilledema or obstructive hydrocephalus, or growth over serial imaging.
Pleomorphic xanthoastrocytoma
Pleomorphic xanthoastrocytomas (PXAs) are rare tumors that account for less than 1% of all astrocytic neoplasms. They have a wide range of age at presentation, from early infancy to the ninth decade of life, with a median of 20 years at the time of diagnosis. Most are classified as WHO grade II and have a relatively favorable prognosis, with 5- and 10-year survival rates of 75% and 67%, respectively. However, between 10% and 23% display a more aggressive behavior with histologically malignant features, and prognosis seems to be worse in males and with increasing age. Anaplastic pleomorphic xanthoastrocytoma, WHO grade III, has been added to the 2016 CNS WHO as a distinct entity. Patients with such tumors have shorter survival times when compared to those with WHO grade II PXAs. Seventy percent to 80% of patients present with seizures.
The imaging features of PXAs are variable. PXAs occur most commonly in the temporal (39%), followed by the frontal (19%) and parietal (14%) lobes. They are overwhelmingly supratentorial, with only 2 cerebellar tumors out of 213 PXAs in the largest single series published to date. These tumors favor a peripheral location and may scallop the inner table of the calvarium, reflecting their slow growth. Most are heterogeneous, and the solid components show avid enhancement and may characteristically abut the meninges ( Fig. 9 ).
Oligodendroglial tumors
The peak incidence of oligodendroglial tumors is between the fifth and sixths decades of life. They are rare in children, in whom they represent 2% to 4% of brain tumors, with the majority being low grade (WHO grade II). In contrast to their adult counterparts, in whom 1p19q codeletions are common and associated with increased chemosensitivity and improved prognosis, such alteration is rare in pediatric oligodendrogliomas. Other molecular features that have been associated with increased overall and progression-free survival in adult oligodendrogliomas, namely Isocitrate dehydrogenase 1 (IDH1) mutations and methylguanine-methyltransferase (MGMT) promoter methylation, appear to have a distinct presentation in the pediatric population. IDH1 mutations, which are frequent in adult oligodendrogliomas, are notoriously rare in children, while MGMT promoter methylation appears to be as common. Oligodendroglial tumors occur most commonly in the frontal lobe, followed by the temporal and parietal lobes. Other locations such as the brain stem, cerebellopontine angle, optic nerve, and spinal cord are rare. The imaging characteristics of oligodendrogliomas are nonspecific, but they tend to be relatively well circumscribed and typically expand the cortex with variable degrees of white matter involvement. Enhancement is less common than in adult oligodendrogliomas and is heterogeneous when present. Susceptibility-weighted imaging (SWI) may be helpful to detect calcification, but this feature also appears to be less common in children ( Fig. 10 ). As opposed to astroglial tumors, where increased perfusion correlates with tumor grade, high rCBV is often found in low-grade oligodendrogliomas. Regardless, perfusion parameters may still have a role in assessing treatment response or disease progression or possibly in predicting malignant transformation.
Ependymoma
Ependymomas constitute 10% of all primary CNS neoplasms in children. Most occur in the posterior fossa, and 40% are supratentorial, half of which are situated within the brain parenchyma. A rare subset of supratentorial ependymomas may selectively involve the cortex and is more commonly associated with seizures. They are found most commonly in the frontal lobes, followed by the parietal lobes. It is believed that parenchymal ependymomas may arise from embryonic ependymal rests trapped during development of the cerebral hemispheres. Due to their parenchymal location, extraventricular ependymomas tend to be larger at presentation than intraventricular ones, which more commonly result in obstructive hydrocephalus. On imaging, ependymomas are usually well circumscribed but heterogeneous tumors that show variable degrees of inhomogeneous contrast enhancement. They have a higher incidence of cysts compared with infratentorial ependymomas; about 50% show areas of calcification, and hemorrhage may occur ( Fig. 11 ). Although their imaging appearance is similar to that of ependymoblastomas and other embryonal tumors, there is suggestion that ependymomas have a higher incidence of cysts that are more often peripherally located and that their enhancement is more commonly inhomogeneous. Similar to their infratentorial counterpart, the solid components of supratentorial ependymomas show low ADC signal due to restricted diffusion. However, ADC values of ependymomas are usually higher than those of embryonal tumors. Perfusion is rarely performed but has been shown to be high with a slow return to baseline. MRS characteristics are nonspecific, with increased choline and reduced N-acetylaspartate, but may be useful for follow-up and determination of tumor recurrence.
Angiocentric glioma
Angiocentric gliomas are now recognized as a distinct subset of glial tumors with uncertain histogenesis but with some degree of astrocytic and ependymal differentiation. Two independent case series were first described in 2005, and these lesions were listed as a new entity in the WHO classification of tumors of the CNS in 2007. Angiocentric gliomas are by far tumors of children and less commonly young adults, although a few cases in older patients have also been described. They are relatively indolent and slow growing (WHO grade I), and most come to attention due to longstanding or intractable seizures. Except for 1 tumor that occurred in the midbrain and was characterized pathologically as angiocentric glioma-like, all other reported cases in the literature have been located in the supratentorial brain, most commonly involving the frontal and temporal followed by parietal and occipital lobes. Angiocentric gliomas are superficial nonenhancing cortical lesions, although a few cases showing subtle to mild enhancement have been reported. Some of them may be intrinsically hyperintense on T1-weighted sequences and have a stalk-like extension to the adjacent ventricle on T2-weighted sequences, features thought to be characteristic but inconsistently present ( Fig. 12 ). A recent study using MRS has found a myoinositol and/or glycine peak in an angiocentric glioma, but for the most part their spectral characteristics overlap with those of other low-grade neoplasms. Information on MR imaging diffusion features is scarce in the literature due to the paucity of published cases. However, no significant restricted diffusion should be expected due to the low-grade histology of this lesion, as shown in 1 reported case where DWI (diffusion weighted imaging) showed facilitated diffusion.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree