(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
When faced with an indeterminate radiological and pathological result, the questions the multidisciplinary team looking after women with this abnormality have to consider are as follows:
Learning Points
Fine needle aspiration cytology and needle core biopsies are more cost-effective methods of diagnosing benign breast disease than open surgical biopsy.
Pathologically indeterminate lesions of B3 category require surgical excision.
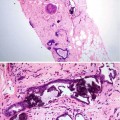
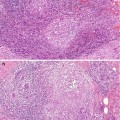
The B3 category lesions include ADH, lobular neoplasia, radial scar, columnar cell lesions, mucocoele-like lesions, microglandular adenosis, papillary and fibroepithelial lesions.
Radiologically B3 lesions are excised to exclude high-risk lesions such as DCIS and invasive cancer.
Excision of a B3 lesion also assists in evaluating the overall risk of malignancy.
At least 30 % of B3 lesions contain a high-risk lesion in the excision biopsy.
4.1 Why Excise Benign Breast Lesions?
When faced with an indeterminate radiological and pathological result, the questions the multidisciplinary team looking after women with this abnormality have to consider are as follows:
(i)
Do they follow up the patient clinically and radiologically and re-biopsy if there is a change in the lesion?
(ii)
Do they repeat the biopsy immediately?
(iii)
Will the repeat biopsy give the answer they want?
(iv)
What if they miss the lesion on repeat biopsy and distress the patient?
(v)
Do they excise the lesion?
Each patient should be managed on an individual basis because there are no correct answers to these questions.
4.2 The Advantages of Nonsurgical Diagnostic Procedures
As part of the triple assessment, nonsurgical procedures such as fine needle aspiration cytology (FNAC) and needle core biopsies are cost-effective when compared with surgical excision biopsy in the management of symptomatic or mammographically detected lesions. Although most savings are apparent with FNAC, studies in the USA revealed that core biopsies produce cost savings of up to 300 % when compared with surgical excision biopsies. Burkhardt and Sunshine (1999) estimated the input resource cost of a needle core biopsy to be US$ 243, compared with US$ 698 for an excisional biopsy. The actual billed costs for the procedures were $ 3,764 and $ 1,496 for excisional and core biopsies, respectively. The costs of surgical biopsies were on average 2.5–3 times those of needle core biopsies (P < 0.001). In a separate study, Liberman et al. (1998) reported that although needle core biopsy was more cost-effective than excisional biopsy, ultrasound-guided biopsies had better cost savings than stereotactic-guided biopsies. In addition to cost savings, nonsurgical diagnostic procedures offer better patient comfort and appropriate planned management. These costs have obviously increased with time.
4.3 Quality Assurance in Surgery of Benign Breast Lesions
The aim of nonsurgical diagnostic procedures is to minimise unnecessary operations for benign lesions. The UK quality assurance guidelines for surgeons in breast cancer screening (NHSBSP 2009) recommend minimising unnecessary open biopsy surgical procedures where there is a definite histological diagnosis of benignity to fewer than 15 per 10,000 women screened in the prevalent round and fewer than 10 per 10,000 women screened in the incident round. Similarly, the European Society of Mastology (EUSOMA) recommends the limitation of the number of unnecessary surgical excisional biopsies and the benign:malignant ratio should not exceed 0.5:1. Excisions due to patients’ requests should be excluded if the benign diagnosis has been confirmed by other tests. For acceptable cosmesis, 90 % of diagnostic biopsies for impalpable lesions that turn out to be benign should weigh less than 30 g (Perry et al. 2001). Besides complications associated with surgery, another reason cited for avoiding surgery for benign disease is tissue scarring, which would make interpretation of subsequent mammograms difficult. However, in a retrospective study comparing women who had undergone surgery for benign disease with those who had not, Slanetz et al. (1998) reported that changes in patients’ breasts due to previous surgery for benign disease rarely created diagnostic problems in interpretation of routine mammography.
4.4 Indications for Surgery in Benign Breast Lesions
The widespread use of nonoperative diagnostic procedures such as needle core biopsies, vacuum-assisted biopsies and fine needle aspiration biopsies can readily differentiate benign breast disease from cancer when the clinical and radiological features concur with the pathological findings. However, with screen-detected abnormalities, pathologists may be unable to exclude malignancy confidently in a needle core biopsy, and this will prompt an excision biopsy. Indications for excision biopsies in lesions that turn out to be benign on histology include the following:
(i)
Discordance between the radiological and histological features
(ii)
Atypical calcification despite benign histological report
(iii)
Calcifications in the posterior area of the breast, which are difficult to access by a needle core biopsy
(iv)
Atypical lesions of unknown malignant potential on histology such as radial scars, papillomas, mucocoele-like lesions, columnar cell lesions, microglandular adenosis, atypical ductal hyperplasia and lobular neoplasia
(v)
Fibroadenomas in patients over 35 years old in some institutions
Open excision biopsies of these atypical lesions are in line with European guidelines for quality assurance in breast cancer screening and diagnosis (Perry et al. 2006). The lesions which prompt an open diagnostic biopsy are considered to be at risk of developing cancer (Shaaban et al. 2002; Schnitt 2003; Hartmann et al. 2005). Manfrin et al. (2009) reviewed 510 open biopsies performed on screen-detected lesions which they classified according to risk of developing cancer as follows:
(i)
Histology 1 (Histo 1) referred to normal breast histology with no lesion detected at biopsy.
(ii)
Histology 2 (Histo 2) category was ‘pure’ benign lesions with no risk of developing cancer, and these lesions included adenosis, ductal micropapillomatosis, fibroadenoma, lipoma, lymphadenitis, mastitis, fibrocystic disease and pseudo-angiomatous stromal hyperplasia.
(iii)
Histology 3 (Histo 3) referred to benign proliferative epithelial lesions with a ‘low risk’ of developing cancer such as mucocoele-like lesions, multiple papillomatosis, papilloma, cellular fibroepithelial lesions and radial scars.
(iv)
Histology 4 (Histo 4) referred to benign proliferative epithelial lesions with a ‘high risk’ of developing cancer: thus, ADH, ALH and atypical columnar cell hyperplasia.
This classification is in variance with that of the UK NHS Breast Screening Programme guidelines for pathology reporting. Although Histo 1 and Histo 2 lesions correspond to B1 and B2 lesions according to the UK NHS Breast Screening Programme guidelines for pathology reporting, Histo 3 and Histo 4 lesions would be collectively classified as B3 lesions, i.e. atypical lesions of uncertain malignant potential. Based on this classification, Manfrin and colleagues reported cancer in 83.7 % (427) of the 510 open biopsies and benign lesions in 16.3 % (83) of the biopsies with a malignant:benign ratio of 5:1. On further analysis of the benign lesions, 4.8 % (4/83) were classified as normal breast histology (Histo 1), 37.4 % (31/83) as pure benign lesions (Histo 2), 31.3 % (26/83) as benign proliferative lesions with low risk of developing cancer (Histo 3), and 26.5 % (22/83) as benign proliferative lesions with high risk of developing cancer (Histo 4). In the latter category, 9 out of the 22 biopsies were reported as ADH. When Manfrin and colleagues compared their results with other studies, they could apply all the four histological categories in two studies by Spencer et al. (1994) and Chew et al. (2006) which reported open biopsies in 68.7 % and 91.3 % Histo 2 categories, respectively. These two studies show very high rates of open biopsies especially in the report by Chew and colleagues because cytology is the main mode of nonsurgical assessment in the Singapore Screening Programme. Spencer and colleagues (1994) reported 22.2 % of Histo 3 lesions, which is not very different from Manfrin, and Chew et al. (2006), 8.7 %. Both Spencer and Chew had no lesions in category Histo 4 which illustrates the difficulties pathologists have in classifying atypical lesions or lesions with the potential to progress to malignancy. In fact in this study, Manfrin highlighted that it is not necessary to further classify the atypical lesions in needle core biopsies into four categories because his Histo 3 and Histo 4 lesions, which would be classified as B3 lesions (atypia of uncertain malignant potential) in the NHS BSP (2006), require surgical biopsy.
Meyer et al. (1998) reviewed 112 non-palpable, mammographically detected lesions, which had been reported as benign on needle core biopsy. Ninety-six of the patients underwent surgical excision, and 16 had repeat needle core biopsies. This additional evaluation was indicated for suspicious mammographic or ultrasound appearances that were discordant with a benign pathological report (24 patients); the lesions were probably missed because the biopsy showed normal breast tissue, non-specific changes or absence of calcification in biopsy (41 patients); pathologist recommendation due to presence of atypical features (31 patients); increased cellularity in a fibroadenoma, suspicious of a phyllodes tumour (nine patients); and five patients with a benign diagnosis at needle core biopsy underwent surgical excision because of history of cancer in the ipsilateral breast. Only two patients requested excision of benign lesions. None of the lesions excised for radiological–pathological discordance were malignant. Of the possibly missed lesions, two invasive carcinomas were identified, one on excision and the other on repeat biopsy. Eleven DCIS, three invasive carcinomas and two phyllodes tumours were identified among the lesions the pathologist recommended for local excision. This study highlights that, despite the high sensitivity and specificity of needle core biopsies in the diagnosis of cancer, in benign disease, there are situations where surgery is unavoidable.
Although the main indication for excising a possibly benign lesion is to exclude malignancy, in some instances patients request excision of radiologically and pathologically benign lesions for psychological peace of mind. In a large multicentre study in Italy, Ciatto et al. (1998) retrospectively reviewed 754 consecutive benign breast biopsies to determine the main indication for surgical excision. The main indication was to exclude cancer in 66.7 % of the patients, followed by an increase in growth of the mammographic lesion in 11.3 % and for cosmetic (3 %) and psychological reasons (8.2 %). Other indications included previous history of breast cancer, family history of breast cancer or contralateral breast cancer. In one centre included in the study, there was a high rate of excision for psychological or cosmetic reasons in 47 % of the patients.
In a separate study, Cant and colleagues (1987) retrospectively questioned 124 women who had undergone surgery for symptomatic, cytologically proven fibroadenoma as to whether they would prefer surgery or conservative treatment for the same diagnosis in future. Twenty-one per cent (26) indicated that they would opt for conservative management for the same problem in the future. When asked whether they would have preferred conservative management for their previously excised masses, only 7 % (8) indicated that they would have opted for nonoperative management. The women assessed in this series had presented symptomatically with a lump, and psychologically it was reassuring to the patient to have the lump removed. Although the numbers are small, these results are similar to the Italian multicentre study, where 8.2 % (62/754) of the women with mammographically and biopsy-proven benign breast lesions requested excisional biopsies for psychological reasons (Ciatto et al. 1998).
4.5 Indeterminate Lesions Excised to Exclude Malignancy
The category of indeterminate B3 lesions can be assigned to both symptomatic and screen-detected breast lesions but with a higher prevalence in the latter where approximately 5 % of the lesions are classified as B3 (El-Sayed et al. 2008; Lieske et al. 2008).
Although the use of directional vacuum-assisted core biopsies may clarify the diagnosis of pathologically indeterminate lesions usually classified as B3 in needle core biopsies (NHS BSP Publication 58, Darling et al. 2000), definitive management of the patient would invariably require an open biopsy. The open biopsy approach raises further questions as to whether the B3 category unnecessarily increases the surgical benign biopsy rate and whether the yield of cancer in the open biopsies justifies the surgery. To determine whether the B3 category increased surgery for benign lesions, Hunt and colleagues (2011) reviewed needle core biopsies from 235 women which had been classified as B3. Twenty-seven (11 %) of the women did not undergo surgery and the remainder, 89 % (208), had open biopsies, and nine of the women who had repeat core biopsies were excluded from the study. This left 199 women who had B3 diagnosis based solely on the original core biopsy. Seventy-three (37 %) of the women were in the prevalent screening round and 126 (63 %) in the incident round. The open biopsy results identified malignancy (invasive and in situ) in 15/73 (21 %) in the prevalent round and 42/126 (33 %) in the incident round (Fisher’s exact test p = 0.038), collectively 29 % (57/199) malignant lesions in B3 reported lesions. There were non-malignant lesions in the remaining 142 (71 %) women with a predominance of radial scars and other benign lesions. In other studies the rates of malignancy in excision biopsies of B3 category were reported as 21 % (Dillon et al. 2007) and 34 % (Lieske et al. 2008). The majority of the cancers in the open biopsies were in situ. The study by Hunt and colleagues (2011) demonstrated the following:
(i)
Excision biopsies in B3 lesions identify malignant lesions in nearly 30 % of the patients.
(ii)
The yield is higher in the incident round as the women are older than the prevalent round.
(iii)
Needle core biopsies underestimate the presence of malignant disease in the presence of atypical proliferations such as ADH and ALH.
(iv)
Where there is uncertainty of the definitive diagnosis of the breast abnormality, excision is justified following multidisciplinary team discussion.
These results are in contrast to those reported by Carder and Liston (2003) who only identified four cancers out of 36 (11 %) B3 category biopsies and the remaining 22 yielded benign lesions in the excision biopsies. Furthermore, BI-RADS Category 3 only yields on 2 % cancers in the excision biopsies (Eberl et al. 2006). The high yield of cancers in the excision biopsies of B3 lesions in the above studies could be explained by a low threshold of reporting B3 lesions in needle core biopsies instead of classifying them as B4. Overall the risk of an open biopsy for a B3 lesion outweighs the need to minimise the number of excised benign lesions as there is evidence that at least 30 % of B3 lesions on biopsy yield cancer on open biopsy.
Jacobs and colleagues (2002) performed a literature review to provide guidelines on which lesions should be excised following an indeterminate diagnosis of a needle core biopsy. The authors recommended surgical excision for ADH, lobular neoplasia (ALH and LCIS), papillary lesions, radial scars, fibroepithelial lesions, mucocoele-like lesions and columnar cell lesions. This section briefly outlines justification for surgical excision in these indeterminate lesions. Berg (2004) advocates a similar approach to the management of these lesions. The radiological and pathological features and any related risk will be discussed in the appropriate chapters.
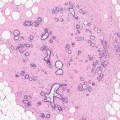
4.5.1 Atypical Ductal Hyperplasia (ADH)
It is well known that patients with ADH have a 4–5 times risk of developing malignancy in both breasts compared to the general population (Dupont and Page 1985). The rationale for excising ADH when diagnosed in a needle core biopsy includes the following:
(i)
The difficulties in distinguishing ADH from low-grade DCIS in a small needle core biopsy can be difficult compared to an excision biopsy.
(ii)
ADH is usually present at the periphery of DCIS, and therefore, diagnosis of ADH in a needle core biopsy is not always representative (Lennington et al (1994).
(iii)
(iv)
Under-diagnosis of cancer with the diagnosis of ADH is more likely if the target lesion has microcalcification than a lesional mass with a 30 % and 5 % carcinoma identified in excision specimens, respectively (Liberman et al. 1997).
(v)
The likelihood of finding carcinoma in the excision biopsy depended on the extent of the ADH in the needle core, with a higher yield of carcinoma if the biopsy had four or more foci of ADH (Ely et al 2001).
Based on these observations, the joint task of the American College of Radiology, American College of Surgeons and College of American Pathologists (Basset et al. 1997) recommended surgical excision on all cases with the diagnosis of ADH on needle core. The same approach is advocated by the UK NHS Breast Screening Programme (2005). However, with the use of the vacuum-assisted biopsies, there are reports of complete removal of small areas of microcalcification with no evidence of a high-risk lesion in the excision biopsies (Villa et al. 2011). Follow-up studies are required to determine whether vacuum-assisted device could be used for therapeutic purposes rather than diagnostic.
4.5.2 Lobular Neoplasia
The term neoplasia is used collectively for atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS). This is because phenotypically and genetically, ALH is identical to LCIS. However, this pathological combination of the terms fails to address the differences in risk associated with malignancy which is 4–5 times and 11 times for ALH and LCIS, respectively (Dupont and Page 1985). Unlike ADH, the management of lobular neoplasia is more controversial because ALH and LCIS are usually identified as incidental findings in the needle core biopsy or excision biopsy during investigation of another lesion such as radial scar, calcifying ADH or DCIS. The incidence of LCIS in needle core biopsies is less than 2 % in most series (Berg et al. 2001b; Liberman et al. 1999a). Local excision of lobular neoplasia is recommended if associated with other high-risk lesions such as radial scar or ADH (Liberman et al. 1999b). The presence of LCIS or ALH in a needle core biopsy that is inconsistent with the radiological findings is also an indication for local excision. Sometimes it is difficult to distinguish ALH and LCIS from low-grade solid DCIS, and local excision would be prudent in these circumstances. E-cadherin immunocytochemistry is a useful adjunct to morphological assessment as this is positive in DCIS and negative in ALH and LCIS (Acs et al. 2001). Amorphous calcification associated with ALH and LCIS has been reported in mammographic lesions diagnosed at needle core biopsy (Berg et al. 2001a). This should prompt an excision biopsy to exclude high-grade DCIS. The current recommendation is that if lobular neoplasia is present in a needle core biopsy, this should be managed by an excision biopsy; but if present in an excision biopsy, no further surgical intervention is necessary (Walker et al. 2012).
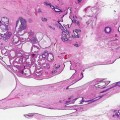
4.5.3 Radial Scar
Radial scars are radiologically indistinguishable from a stellate invasive cancer. Previously it was debatable as to whether radial scars were a risk factor for cancer or not. There is now a body of evidence that women with radial scars have a twofold increase in cancer risk in both breasts (Jacobs et al. 1999) and the risk of cancer is higher in larger radial scars (Sloane and Mayer 1993). In the later study, Sloane and Mayer reported a 30 % prevalence of carcinoma in radial scars more than 6 mm in size. Of significance in the series reported by Sloane and Mayers was that the carcinomas were present at the periphery of the radial scars and therefore invariably not sampled in the needle core biopsies which target the fibro-elastotic core of the radial scar. In a separate study of 40 women with lesions mammographically suggestive of radial scars, Frouge and Colleagues (1995) reported the presence of carcinoma in 8 (28.6 %) of 28 pathologically confirmed radial scars. Based on these findings, it is doubtful that any multidisciplinary team looking after women with lesions radiologically and pathologically suggestive of a radial scar will have alternative management but recommend excision biopsies. However, Brenner and colleagues (2002) advocate conservative management with the use of a directional vacuum-assisted device, if the diagnosis of a radial scar can be made in needle core biopsies in at least 12 specimens. This only applies if there is no associated ADH or if the pathological features are in keeping with the radiological features.
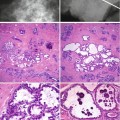
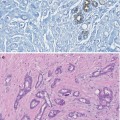
4.5.4 Columnar Cell Lesions (CLLs)
When the first edition of the book was written, there was little information regarding the cancer risk associated with columnar cell lesions and how to manage if identified in a needle core biopsy. There is also a better understanding of the terminology than it was in the first edition of this book. The pathologists are also becoming aware of this lesion, and more information is now available in the literature regarding the biological nature of these lesions.
Columnar cell lesions are a spectrum of lesions characterised by architectural and cytological alteration of the terminal duct of lobular units which becomes irregular or mildly dilated and lined by a layer of epithelium with apical snouts. The lesions are usually detected mammographically due to the presence of microcalcification but are also invariably present in biopsies sampled for investigation for other breast lesions, benign or malignant.
Columnar cell lesions have been reported in the literature under different names (Chap. 11), but there is now an acceptable unifying terminology based on the classification by Schnitt and Vincent-Salomon (2003). The spectrum of the lesions is classified into columnar change (CCC), columnar cell change with hyperplasia, columnar cell change with atypia and columnar cell hyperplasia with atypia with the advanced end of the spectrum being DCIS. Columnar cell change with atypia and columnar cell hyperplasia with atypia were collectively classified as flat epithelial atypia (FEA) by the WHO (Tavassoli and Devilec 2003). When there is prominent architectural alteration, the designation of atypical ductal hyperplasia applies (CCL-ADH). CCC with atypia and columnar cell hyperplasia with atypia, if present in a needle core biopsy, will fall into the B3 category, i.e. atypia of uncertain malignant potential (Walker et al. 2012). Evaluation of the published results of the excision biopsies following the diagnosis of FEA on needle core biopsies reported the presence of DCIS and lobular neoplasia up to 22 % and 36 % of cases, respectively (Jar-Lazaro et al. 2009). DCIS or invasive carcinoma was reported in other studies where excision biopsies were undertaken following the diagnosis of FEA on needle core biopsy (Brogi and Tan 2002; Bonnet et al. 2003; Martel et al. 2007).
The biological significance of columnar cell lesions is still evolving, and further information is required on long-term follow-up of these lesions. Based on the currently available data, the following management is advocated on finding CCL in needle core biopsies:




(i)
CCCs and columnar cell hyperplasia without atypia should be classified as B2 lesions in the same category as fibrocystic change following examination of multiple levels (Walker et al. 2012).
(ii)
CCLs with atypia (FEA) and more complex lesions (ADH) should be classified as atypical proliferations of uncertain malignant potential (B3) and, following multidisciplinary team discussion, recommend surgical excision (Jar-Lazaro et al. 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree