Fig. 19.1
Illustration (a) of a standard pancreaticoduodenectomy. Intraoperative photograph (b) demonstrates the gastrojejunostomy performed as part of this procedure
Pyloric-preserving pancreaticoduodenectomy is a modified PD that preserves the gastric antrum, pylorus, and the proximal 2–3 cm of the duodenum, which is anastomosed to the jejunum to restore gastrointestinal continuity (Fig. 19.2a, b).
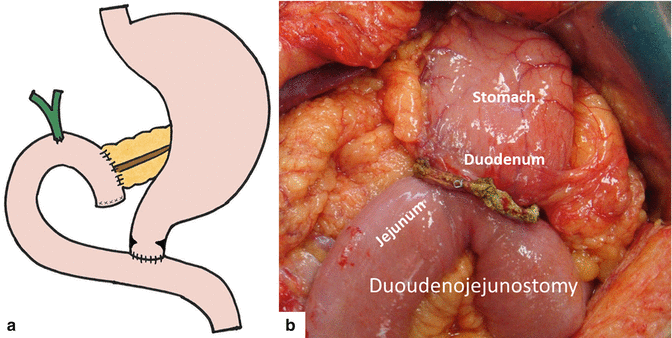

Fig. 19.2
Illustration (a) of a pyloric-sparing pancreaticoduodenectomy. Intraoperative photograph (b) demonstrates the duodenojejunostomy performed as part of this procedure
Pyloric-preserving pancreaticoduodenectomy is usually performed in order to improve the postoperative nutrition status and decrease the incidence of postoperative dumping, marginal ulceration, and bile reflux gastritis.
In experienced hands, a pancreaticoduodenectomy carries a mortality rate between 3 and 5 %.
19.2.1 Indications
Malignant Lesions
Pancreatic ductal carcinoma
Malignant neuroendocrine tumor
Malignant intraductal papillary mucinous neoplasm (IPMN)
Ampullary carcinoma
Common bile duct carcinoma
Duodenal carcinoma
Pancreatic metastasis
Benign Conditions
Benign periampullary neoplasm not amenable to local resection with ampullectomy or enucleation
Neuroendocrine tumor
Benign duodenal neoplasms
Pancreatic head trauma
Borderline IPMN
Chronic pancreatitis
Practical Pearls
Pyloric-preserving pancreaticoduodenectomies have similar long-term survival outcomes as do conventional pancreaticoduodenectomies but are associated with shorter operative times and decreased blood loss.
Cholecystectomy is performed as part of PD surgery. If the gallbladder is left in place, there is a significant incidence of stones and cholecystitis. This occurs due to bile stasis secondary to loss of hormonal (cholecystokinin) and neuronal (vagal) stimulation of the gallbladder contraction.
19.2.2 Preoperative Considerations
Imaging
High-resolution pancreatic protocol CT scan (triple phase with contrast) should be used to characterize the size, location, and relation of the tumor to the surrounding organs and vascular structures.
Endoscopic ultrasound is a great tool used to characterize the tumor (solid vs. cystic). Tissue and fluid samples are taken to establish pathologic diagnosis and malignant potential.
MRCP and ERCP are imaging modalities to evaluate involvement of the main pancreatic and common bile ducts by the tumor.
Bowel Preparation
Clear liquid diet beginning 1 day prior to surgery with gentle bowel preparation
Practical Pearl
Thromboprophylaxis is recommended for patients undergoing resection of a pancreatic mass due to the moderate to high risk for thromboembolism.
19.2.3 Operative Technique (Figs. 19.3–19.22)
Position
Patient is placed in a supine position.
Access
A midline incision or bilateral subcostal incision is performed.
Self-retaining retractors are placed following manual inspection of the four quadrants of the abdomen for distant metastasis to nearby structures or carcinomatosis.
Inspection of the transverse mesocolon and ligament of Treitz should be done to detect direct extension of the tumor.
Exposure and Mobilization
A Kocher maneuver (Fig. 19.3) is performed by incising the peritoneum from the lateral edge of the duodenum, allowing the duodenum and the pancreatic head to be reflected medially. This maneuver allows the surgeon to palpate the tumor and detect the tumor’s separation from the superior mesenteric artery (SMA) and declare resectability of the mass.
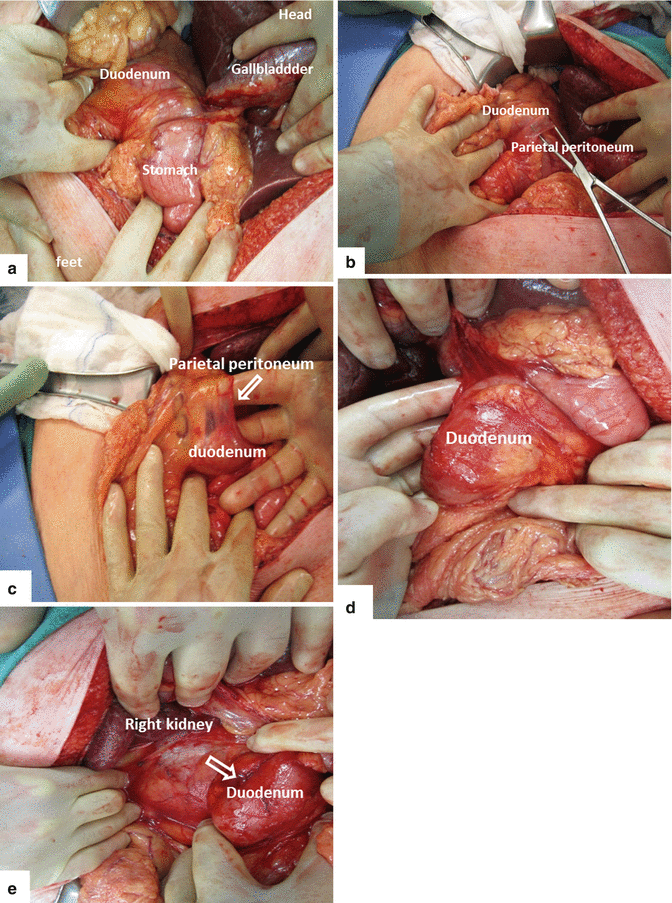
Figs. 19.3
Kocher manuever (a–e)
Figs. 19.4
Entering the lesser sac
Figs. 19.5
Gastroepiploic vein ligation
Figs. 19.6
Pancreatic dissection
Figs. 19.7
Gastrohepatic ligament dissection
Figs. 19.8
Dissection along the lesser curvature of the stomach (a, b)
Figs. 19.9
Dissection of the duodenum (a, b)
Figs. 19.10
Replaced right hepatic artery from the SMA
Figs. 19.11
Transection of the pancreas (a, b)
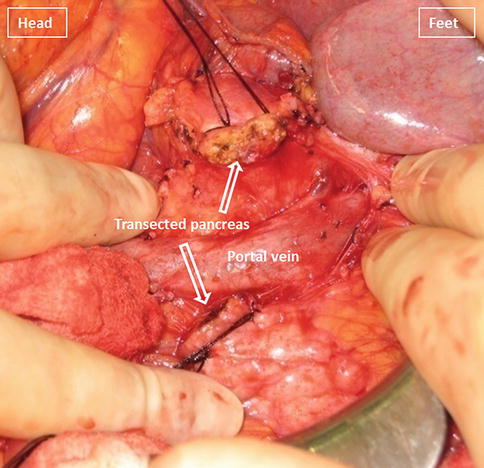
Figs. 19.12
Transected neck of the pancreas
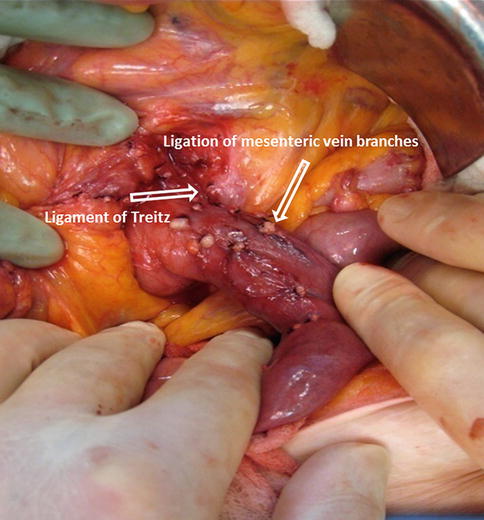
Figs. 19.13
Ligation of mesenteric vessels
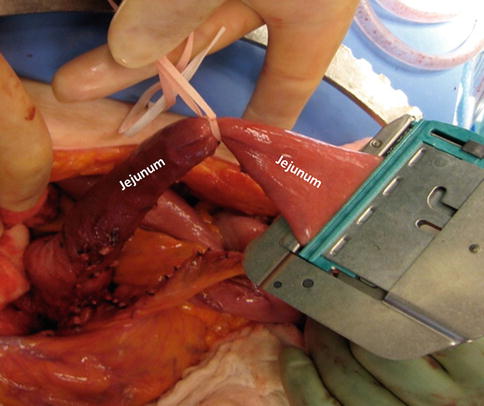
Figs. 19.14
Transection of the jejunum
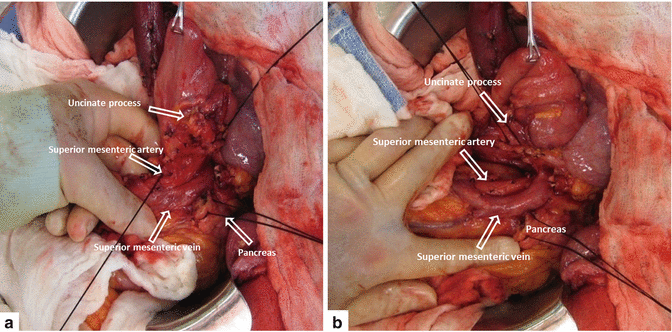
Figs. 19.15
Ligation of vascular branches from PV, SMV, and SMA (a, b)
Figs. 19.16
Transected CBD
Figs. 19.17
Skeletonized specimen
Figs. 19.18
Delivering the jejunum (a–c)
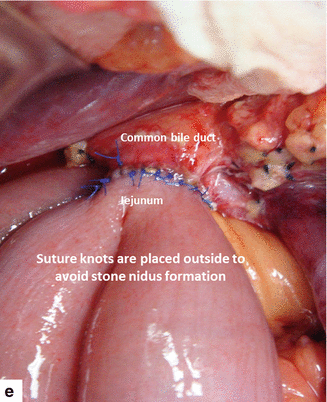
Figs. 19.19
Performing (a–c) and completed hepatojejunostomy (d, e)
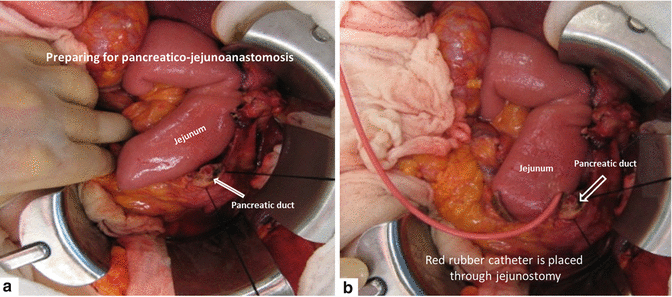
Figs. 19.20
Pancreaticojejunostomy preparation (a, b)
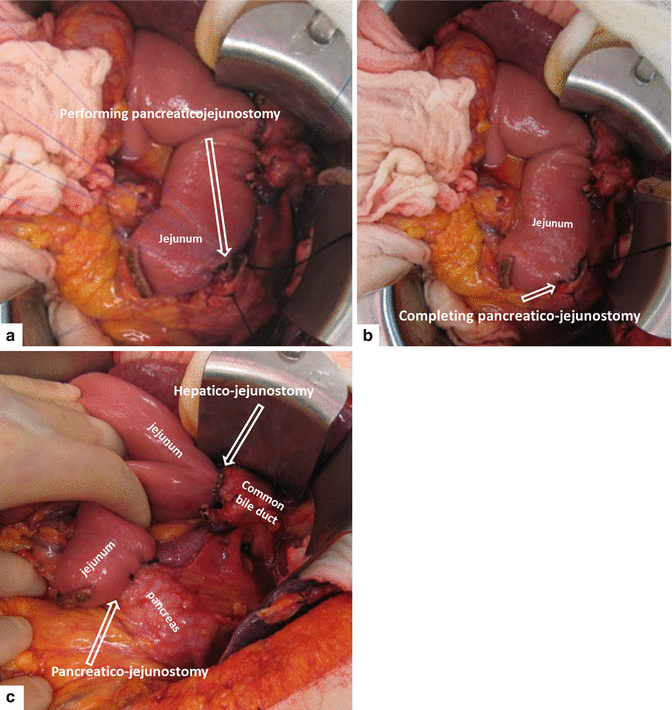
Figs. 19.21
Performing (a, b) and completed pancreaticojejunostomy (c)
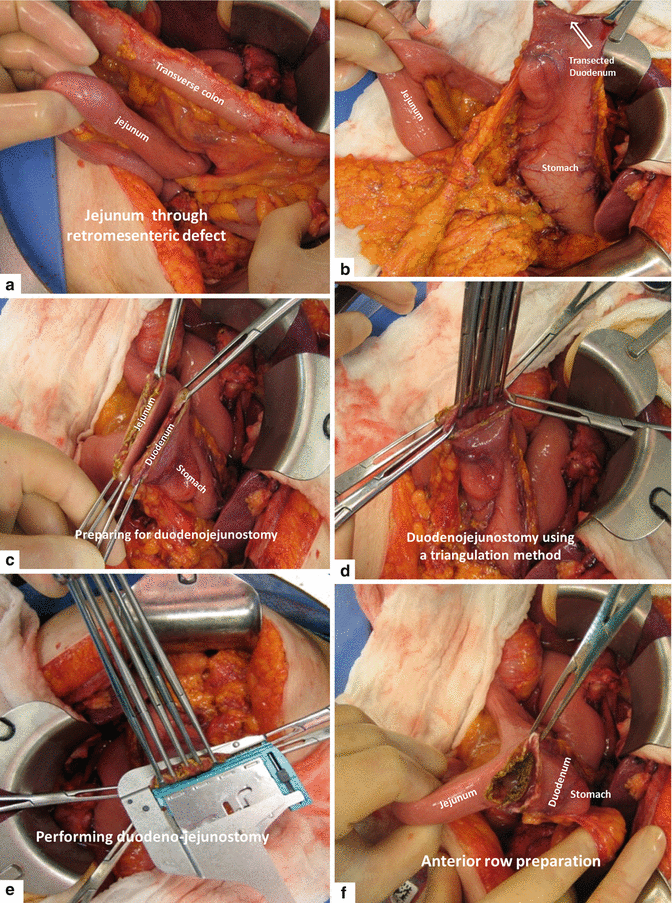
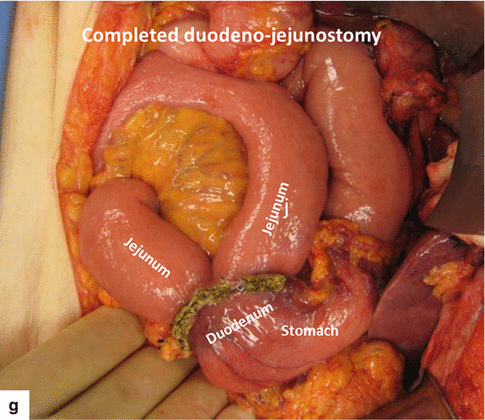
Fig. 19.22
Performing (a–f) and completed duodenojejunostomy (g)
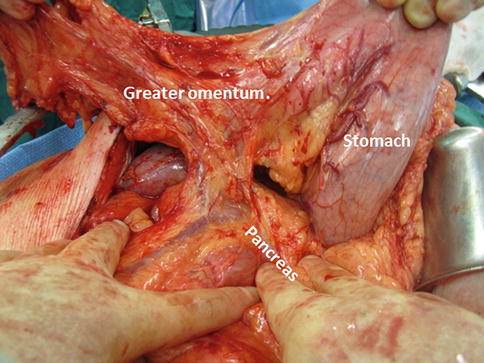
The stomach is retracted superiorly and the lesser sac is entered through the gastrocolic ligament. An alternative is to reflect the gastrocolic omentum with the stomach (Fig. 19.4).
Further dissection of the omentum, extending from the splenic flexure to the hepatic flexure, is made. The branches of the gastroepiploic vessels should be left intact to allow adequate perfusion of the stomach and omentum.
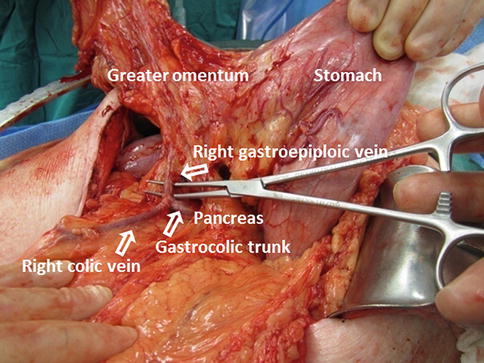
The right gastroepiploic vein is identified, ligated, and divided from the gastrocolic trunk, giving exposure to the superior mesenteric vein (Fig. 19.5).
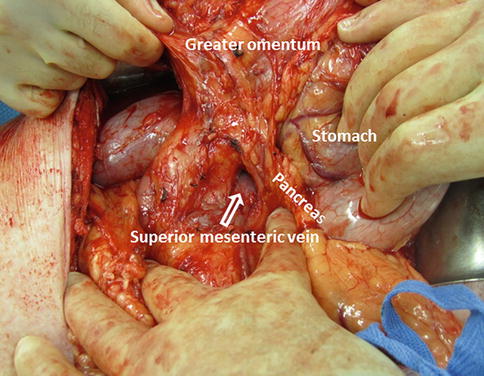
The inferior margin of the pancreas is dissected and the superior mesenteric vein (SMV)-portal vein (PV) confluence is identified (Fig. 19.6).
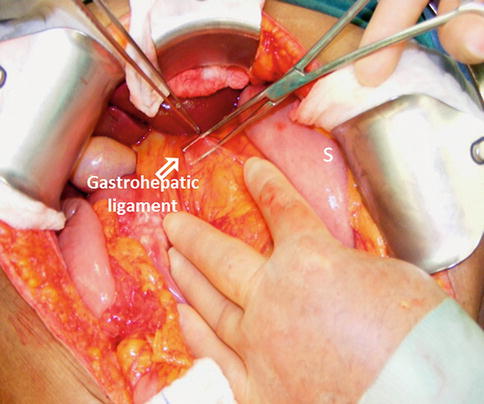
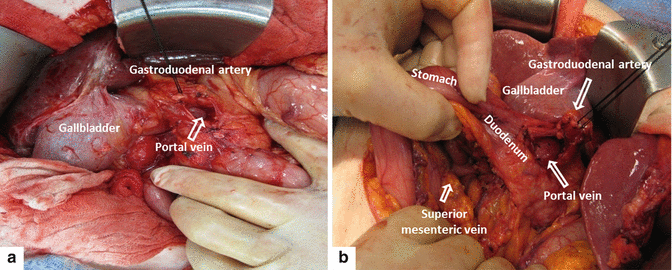
The gastrohepatic ligament is identified and dissected (Fig. 19.7) until the right gastric artery is isolated then ligated and divided. Blunt dissection along the lesser curvature of the stomach is performed until the gastroduodenal artery (GDA) is identified. This artery is isolated and test clamped (Fig. 19.8) to confirm adequate flow through the proper hepatic artery before its ligation and division. It allows exposure and dissection of the portal vein.
In preparation for a pyloric-sparing procedure, the duodenum is then dissected a couple centimeters beyond the GDA and is divided with a linear cutter stapler 2–3 cm distal to the pylorus (Fig. 19.9).
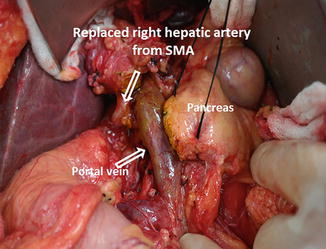
In approximately 10–15 % of patients, a right hepatic artery originates from the superior mesenteric artery (Fig. 19.10); this anatomic variant is suggested by palpating a pulse on the right of the bile duct. Its ligation should be avoided.
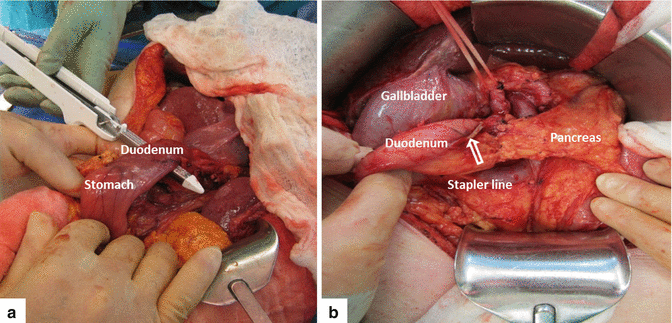
Transection of the Pancreas
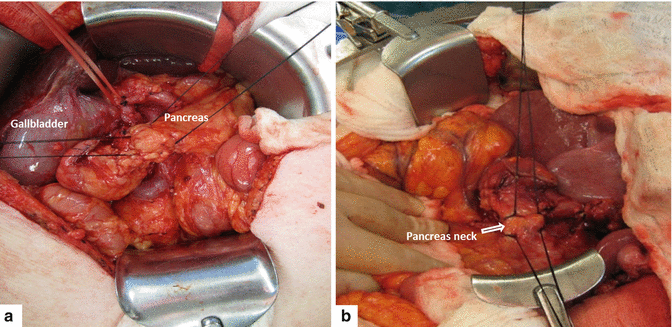
Transection begins by applying a 2-0 silk suture to each of the four corners of the neck of the pancreas in a figure eight fashion (Fig. 19.11). This is used to ligate the superior and inferior transverse pancreatic vessels of the neck of the pancreas.
Using electrocautery, the neck of the pancreas is transected between the figure eight sutures (Fig. 19.12). During this step, it is important to identify the location of the pancreatic duct in order to avoid unintentional ligation at the time of the pancreaticojejunostomy.
The ligament of Treitz is then identified at the base of the transverse mesocolon and a segment of approximately 10 cm of jejunum is dissected along with the ligation of the mesenteric branches (Fig. 19.13).
An umbilical tape is placed around the distal portion of the freed jejunum and a linear cutting stapler is used to transect the jejunum (Fig. 19.14).
The transected portion of the jejunum is then passed retrocolic or retromesenterically and positioned over to the right of the abdomen.
The pancreatic head and uncinate process are separated from the right lateral border of the PV, SMV, and SMA (Fig. 19.15). All vascular branches, as they are identified, are ligated.
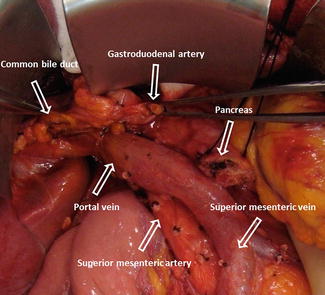
The common bile duct is identified and transected (Fig. 19.16). A sample of bile should be sent for laboratory analysis for culture and sensitivity. This is particularly important if a biliary stent was used.
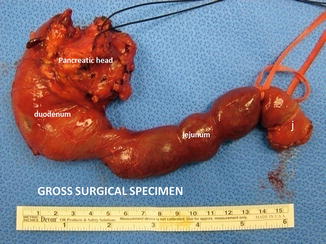
The skeletonized specimen is removed from the field and marked to identify the bile duct, pancreatic neck, duodenum, and uncinate margins (Fig. 19.17). The SMV-PV groove and neck margins are inked and checked for tumor involvement. If involved, one may extend resection into the body of the pancreas. If the margins are still involved, a total pancreatectomy, with or without splenectomy, is performed.
After the pancreaticoduodenectomy, a cholecystectomy is performed.
Reconstruction
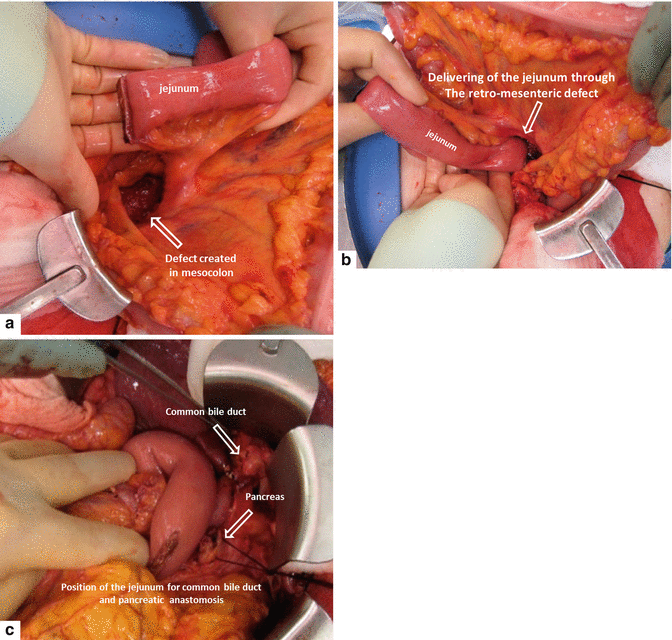
The jejunum is delivered to the right upper quadrant through a created defect in the transverse mesocolon or through a retromesenteric defect (Fig. 19.18). Careful attention is paid to avoid twisting the mesentery.
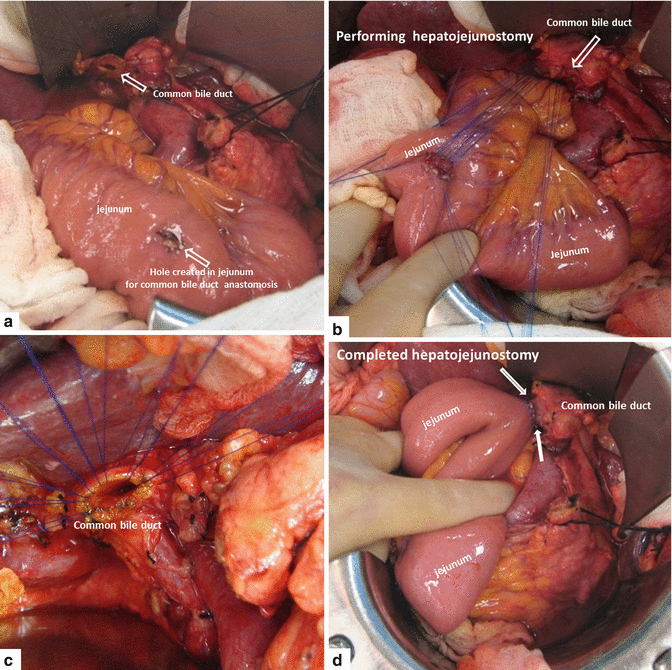
A small enterotomy is created in the jejunum using the electrocautery and the mucosa is everted using 5-0 vicryl sutures, Hepaticojejunostomy is then performed in an end-to-side fashion using interrupted 5-0 prolene sutures in a single layer (Fig. 19.19).
Using electrocautery, a jejunostomy is created in the distal blind end of the jejunum. A red rubber catheter is placed inside to facilitate the anastomosis (Fig. 19.20). A duct dilator can be used to expand the main pancreatic duct to an adequate size.
A pancreaticojejunostomy is performed as an end-to-side duct to mucosa anastomosis using a single layer of 5-0 prolene sutures. The outer layer is reinforced with interrupted 5-0 prolene sutures (Fig. 19.21).
The anastomosis should be inspected for evidence of bleeding and irrigated using copious amounts of saline.
The previously transected duodenum and stomach are brought back into the field in preparation for an antecolic duodenojejunostomy using a triangulation staple technique. This method uses three loads of a linear stapler (Fig. 19.22).
Once the anastomoses are completed, a closed suction drain is placed near the choledochojejunostomy and pancreaticojejunostomy to control any possible leak.
19.2.4 Postoperative Care
Patients are transferred overnight to the surgical intensive care unit (SICU). Patients should remain in the SICU if they manifest cardiac or respiratory problems.
The nasogastric tube (NGT) is maintained until the patient regains gastric function.
Initially, a liquid diet begins 24 hours after the NGT is removed; if tolerated, regular diet is started 24 hours later.
Surgical drains are not removed until a low fluid output and low fluid amylase levels are detected and checked once diet is tolerated.
The patient is discharged from the hospital, in an average of 8–10 days after surgery.
19.2.5 Postoperative Complications
19.2.5.1 Delayed Gastric Emptying (Fig. 19.23)
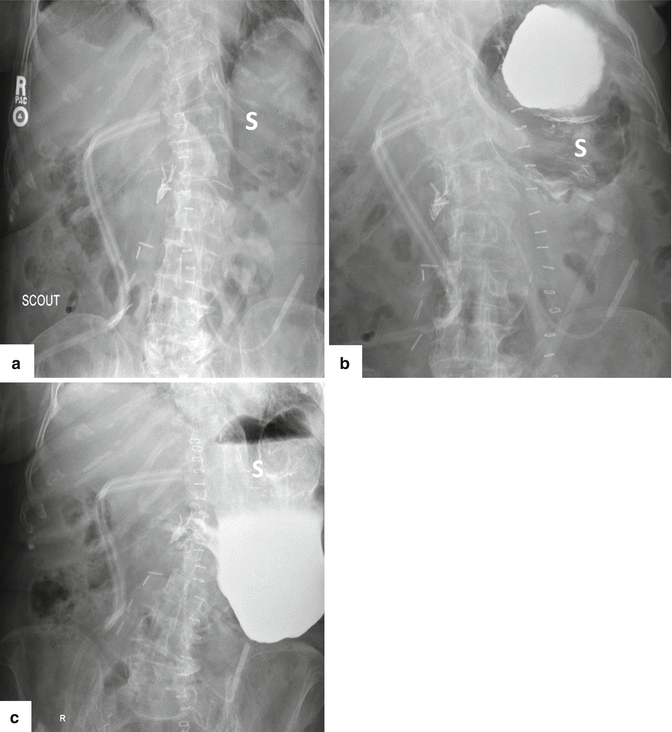
Fig. 19.23
Pyloric-preserving pancreaticoduodenectomy complicated by gastroparesis. A 79-year-old male with history of pancreatic carcinoma who underwent a Whipple procedure 7 days prior. The patient was unable to tolerate feedings. Supine plain film of the abdomen (a) shows a distended stomach (S). Note a surgical drain and surgical staples in the mid abdomen. The patient underwent an upper gastrointestinal series. This study showed lack of gastric peristalsis and poor emptying of the barium from the stomach. Abdominal films obtained 1 hour (b) and 5 hours (c) later show persistent barium retention in the stomach
Leading procedure-related morbidity.
Mostly resolves spontaneously.
Pancreatic leaks and collections may be the etiology of prolonged gastric atony.
Management: nasogastric decompression; attention to nutritional support; prokinetic agents, such as “Reglan” or intravenous erythromycin (motilin agonist); and watchful waiting. If an abdominal collection is detected, it should be drained percutaneously.
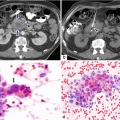
19.2.5.2 Pancreatic Leakage (Fig. 19.24)
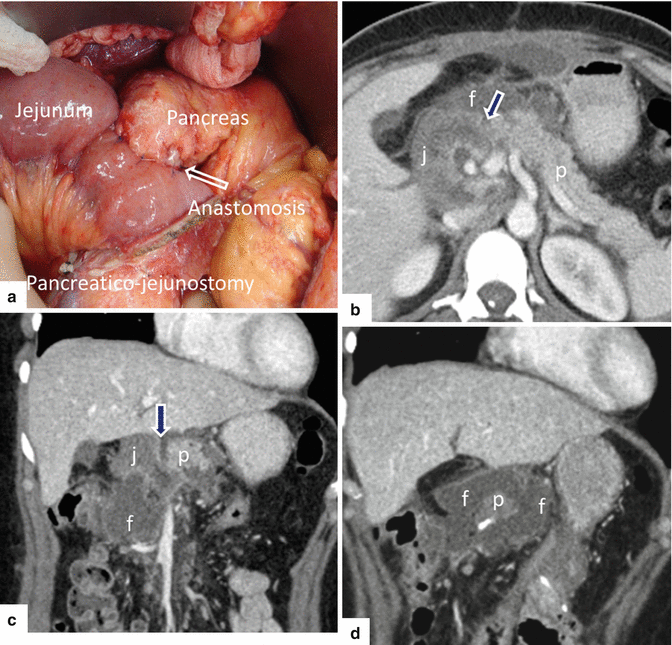
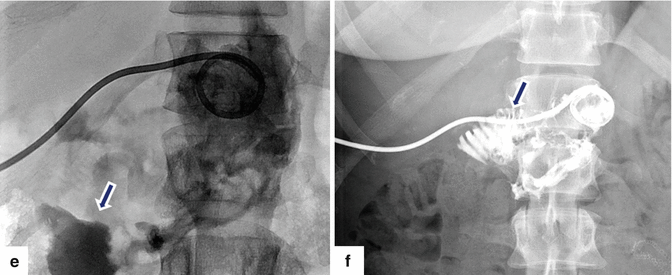
Fig. 19.24
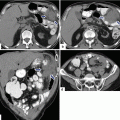
Pancreaticoduodenectomy complicated by a pancreaticojejunostomy leakage. A 48-year-old female with history of cystic mass in the head of the pancreas, who underwent a pyloric-sparing Whipple procedure 10 days prior. Interoperative photograph of a non-complicated pancreaticojejunostomy (a). The patient was discharged home and came back to the hospital with abdominal pain and fever. CECT axial (b) and coronal (c, d) images reveal a complex peripancreatic fluid collection (f) adjacent to the pancreaticojejunostomy (arrows) suggestive of a pancreatic leak. This collection was drained percutaneously under CT guidance with a 10 French APD catheter. An abscessogram performed a week later (e) reveals a peripancreatic collection connecting with the jejunum (arrow). The output from the drainage catheter decreased progressively. Abscessogram repeated 4 weeks later (f) demonstrates significant decrease in size of the peripancreatic fluid collection but persistent connection with the afferent jejunal limb (arrow)
Suspected: when the postoperative amylase content is greater than three times the normal serum amylase content.
These leaks may be associated with fever, leukocytosis, sepsis, or hemorrhage.
Management:
Most leaks may run a benign course, requiring just maintenance of intraoperatively placed drains. Undrained leaks require a placement of percutaneous catheter drainage with CT guidance
Pancreatic enzymes are given to suppress output in patients that are on a p.o. diet.
Somatostatin may be needed to decrease the pancreatic fistula output.
19.2.5.3 Duodenojejunostomy or Gastrojejunostomy Leakage (Fig. 19.25)
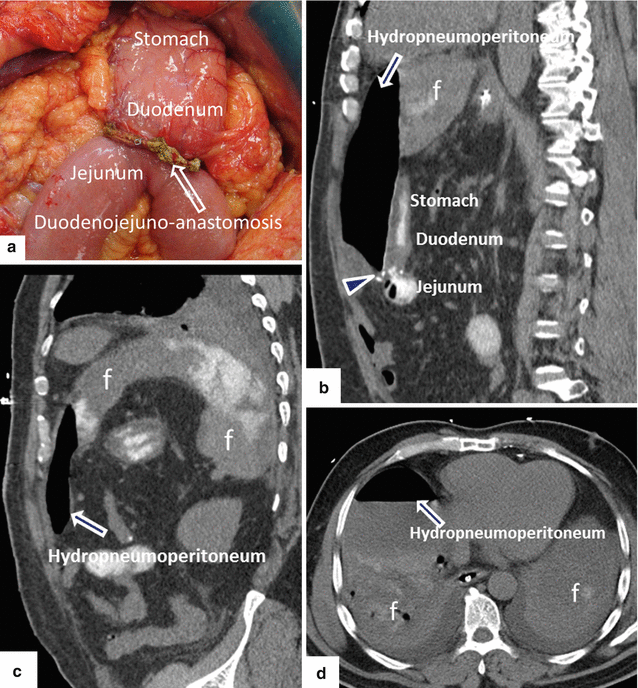
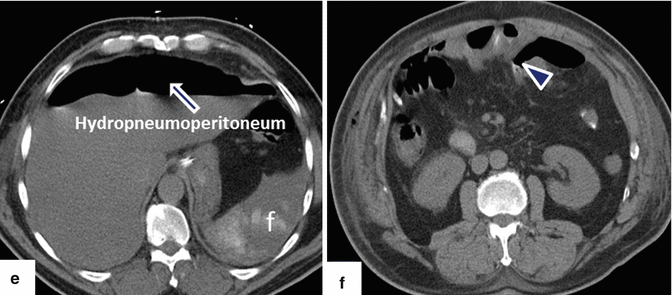
Fig. 19.25
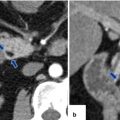
Pancreaticoduodenectomy complicated by duodenojejunostomy leakage. A 66-year-old male with a history of intraductal papillary mucinous carcinoma (IPMN). The patient underwent a pyloric-sparing Whipple procedure. The patient did well in the initial postoperative period; however, on postoperative day 3, the patient became tachycardiac. The patient underwent a CECT of the chest and an echocardiogram to rule out pulmonary emboli; both studies were negative. Subsequently, the patient had an abdominal CT. Intraoperative photograph of a non-complicated duodenojejunostomy (a). CECT sagittal (b, c) and axial (d–f) images demonstrate extravasation of oral contrast at the duodenojejunostomy staple line (arrowheads). Note the large collections with extravagated oral contrast in both subphrenic spaces (f) and the presence of a large hydropneumoperitoneum. The patient was immediately taken back to the operating room for further exploration
Very rare.
These leaks are associated with fever, leukocytosis, sepsis, abdominal distention, and/or acute peritonitis.
Management: surgical reintervention
19.2.5.4 Biliary Leak
Bile leaks from hepaticojejunal anastomosis are very rare.
Suspected by the appearance of bile in the drainage fluid.
The drain should be left in place until the leaks stops. If it is still present when the patient is ready for discharge, the patient can go home with the drain in place.
A transhepatic biliary drainage may be used to decrease volume leak and promote faster healing.
19.2.5.5 Intra-abdominal Abscess (Fig. 19.26)
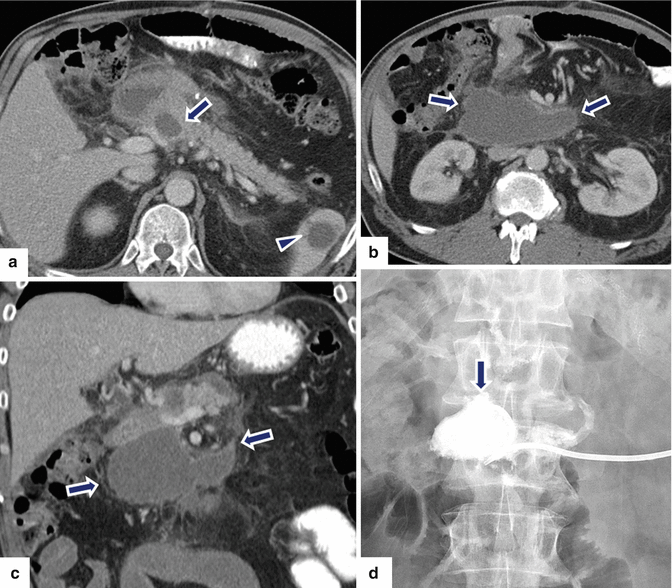
Fig. 19.26




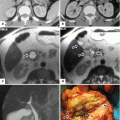
Pancreaticoduodenectomy complicated by an intra-abdominal abscess. A 62-year-old male with history of pyloric-preserving pancreaticoduodenectomy 2 weeks prior. He came back to the ER with fever and abdominal pain. CECT axial (a, b) and coronal (c) images show a large retroperitoneal collection between the mesenteric vessels, aorta, and inferior vena cava (arrows) and an incidental splenic cyst (a, arrowhead). 100cc of purulent material was aspirated from this collection using an 8-French APD catheter placed under CT guidance. Cultures of the aspirated fluid revealed heavy growth of E. coli organisms. The patient was treated with antibiotics as well as aspiration and flushing of the catheter with saline solution every 6 h. Abscessogram (d) performed a week later shows a well-organized cavity in the pancreatic bed (arrow)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree