Etiology
The pathogenesis of systemic lupus erythematosus (SLE) involves genetic and environmental factors, hormonal influences, and cell-mediated responses. In SLE, B lymphocytes lose self-tolerance and inappropriately produce autoantibodies. Serologic positivity for antinuclear antibodies (ANAs) is found in nearly all patients, but anti–deoxyribonucleic acid (DNA) antibodies, although less frequent than ANAs, are far more specific for SLE. A high titer of anti–double-stranded DNA (dsDNA) is considered the best marker of disease activity in SLE.
Genetic evidence for a hereditary tendency comes from the concordance rate in monozygotic twins (≈25% vs. 2% in dizygotic pairs). The search for lupus susceptibility genes has uncovered considerable genetic variance, and human leukocyte antigen associations vary according to ethnicity.
Apoptosis (the process of cell death precipitated by various insults) is thought to be crucial to the development of SLE. The autoimmunity of SLE is suspected to be triggered by exposure to both Epstein-Barr virus and cytomegalovirus. Sex hormones may have a role in developing or modulating SLE because the disease primarily affects women of childbearing age and is exacerbated by estrogen in lupus-prone mice.
Prevalence and Epidemiology
The prevalence of SLE ranges from 17 to 48 per 100,000 population. Most patients are female (female-to-male ratio, 8 : 1) and between the ages of 15 and 45 years. During childhood and after menopause the female-to-male ratio is closer to 2 : 1. Survival statistics have improved in patients with SLE, and in a large cohort of European patients, 92% were found to have survived for 10 years after onset of the disease. The highest mortality is in patients with infections, renal disease, non-Hodgkin lymphoma, and lung cancer. Mortality risk is greatest with female gender, younger age, disease duration of less than 1 year, and African-American ethnicity. Overall, the most frequent causes of mortality in SLE are active disease, thrombotic complications, and infections.
Clinical Presentation
The most frequent symptoms of SLE are arthritis, malar rash, photosensitivity, and neurologic involvement. In the first 5 years after a diagnosis of SLE, the most common cause of death is infection, but after this period thrombotic disease becomes the most frequent cause of mortality. The diagnosis of SLE is often elusive, and it may take up to 2 years from the first manifestations of the disease to make a firm diagnosis of SLE.
Recurrent pleuritic chest pain due to serositis is the most common thoracic symptom in patients with SLE. Pulmonary embolism is another common cause of pleuritic chest pain, particularly in those who have antiphospholipid syndrome (see later). Finally, pericarditis and/or pericardial effusion may result in chest pain.
Lower respiratory tract infections can be caused by either common or opportunistic pathogens because patients are frequently immunosuppressed. Patients with lupus can have fulminant respiratory failure; important causes include opportunistic infections, drugs, impaired renal or cardiac function, diffuse alveolar hemorrhage, or lupus pneumonitis.
In autopsy studies chronic pulmonary disease is common in SLE, with some form of pleural or parenchymal disease found in up to 98% of patients; however, chronic pulmonary involvement may be mild and asymptomatic in many patients. “Shrinking lung” syndrome—low lung volumes possibly caused by a combination of diaphragmatic weakness and chest wall restriction—can cause progressive chronic dyspnea that can occasionally be fatal.
Antiphospholipid syndrome is common in SLE patients, characterized by vascular thrombosis or obstetric complications (or both), thrombocytopenia, and the presence of certain antibodies, such as anticardiolipin and lupus anticoagulant.
Pulmonary hypertension affects up to 8% of patients with SLE, with half of cases classified as primary and half as secondary pulmonary hypertension in one cohort. Patients with SLE are at increased risk for atherosclerosis, and SLE is an independent risk factor for stroke and myocardial infarction. Women 18 to 44 years of age with SLE are twice as likely as those without lupus to have a myocardial infarction or stroke, and heart failure is nearly four times as likely to develop.
Renal disease affects about a third of patients with SLE and remains one of the most dangerous life-threatening complications. Gastrointestinal involvement commonly results in nonspecific abdominal pain and dyspepsia. Hepatosplenomegaly can fluctuate with disease activity, and although mesenteric vasculitis is very rare, it can be life threatening.
Arthralgia and myalgia occur in most patients with SLE; the classic “Jaccoud arthropathy” affects the hands of up to half of patients with SLE and is characterized by reducible, nonerosive joint deformities with preservation of hand function. Skin involvement in SLE is very common and includes the classic malar rash, discoid rash, and generalized photosensitivity. Sun sensitivity, mouth ulcers, and dry mouth can occur.
Hematologic features include normocytic normochromic anemia, thrombocytopenia, and leukopenia.
Manifestations of neuropsychiatric lupus can include headache, seizures, and psychiatric diagnoses, including depression, psychosis, and neuropathy.
Pathophysiology
Pathology
Other than infection, “lupus pneumonitis” has been described as the most common acute lung disease in lupus patients. However, the incidence of lupus pneumonitis has likely been overestimated and may be lower than 4% overall; although the condition is characterized by diffuse alveolar damage, many cases of pneumonia and aspiration were found to have been mistaken clinically for lupus pneumonitis in a large autopsy series. When it occurs, lupus pneumonitis has a high mortality of almost 50%. The frequency of acute lupus pneumonitis in hospitalized patients with lupus is up to 6%, and the outcome of patients with diffuse alveolar damage is either healing and fibrinolysis or parenchymal scarring; chronic fibrotic lung disease occurs in a subset of patients.
In a description of surgical lung biopsy specimens from two patients with SLE, one demonstrated usual interstitial pneumonia and the other follicular bronchiolitis with a minor component of cellular nonspecific interstitial pneumonia. The proportion of patients with SLE who have primarily fibrotic lung disease has been estimated to be less than 10%. Organizing pneumonia is not a common pulmonary manifestation of SLE.
Diffuse alveolar hemorrhage is rare in SLE but has a high mortality rate. Diffuse alveolar hemorrhage in SLE is pathogenetically similar to lupus renal microangiopathy. There are isolated case reports of lymphoid interstitial pneumonia in SLE, usually in the presence of an overlap with Sjögren syndrome.
The myopathy of SLE can affect the diaphragm and result in a raised diaphragm and basal atelectasis, the so-called shrinking lung syndrome. There has been much debate concerning the pathogenesis of this phenomenon, and the cause remains controversial. In one study the majority of patients with “shrinking lung” syndrome had normal neuromuscular diaphragmatic function.
There is an association between SLE and certain neoplasms, particularly hematologic malignancies and non-Hodgkin lymphoma and possibly lung and hepatobiliary cancer.
Lung Function
A range of pulmonary function abnormalities occur in patients with SLE, and as a generalization, abnormal pulmonary function test results are reported in approximately two-thirds of patients with SLE.
Manifestations of the Disease
Radiography
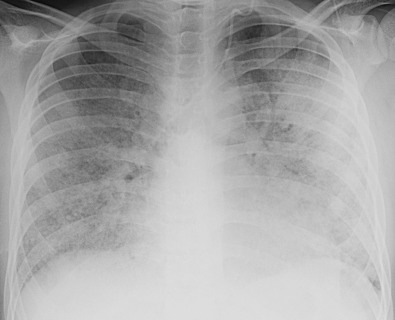
The most common radiographic abnormalities in SLE are the presence of bilateral pleural effusions or pleural thickening ( Fig. 41.1 ) and enlargement of the cardiopericardial silhouette because of pericardial effusion or cardiomegaly. The most common pulmonary finding is consolidation secondary to infection. Other acute manifestations of SLE include pulmonary embolism; acute lupus pneumonitis, which occurs in less than 4% of SLE patients; and pulmonary hemorrhage, which occurs in less than 2% of patients.
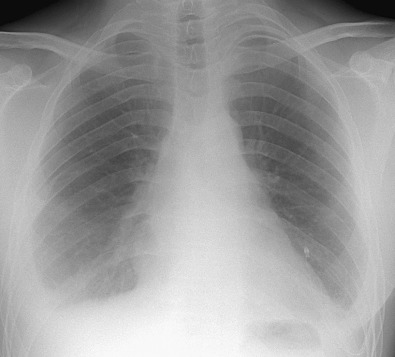
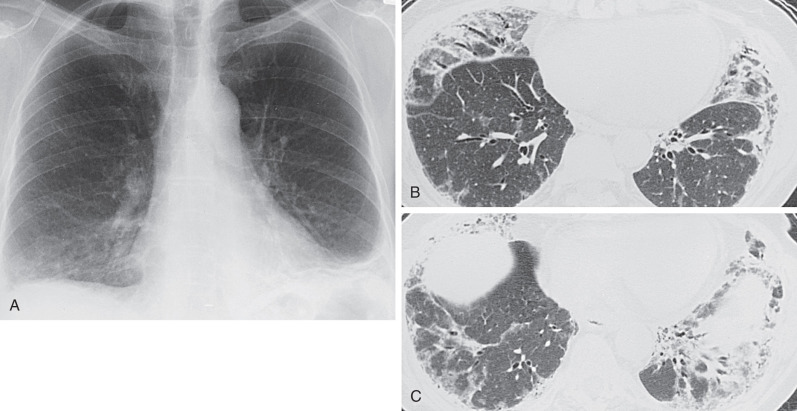
In acute lupus pneumonitis the chest radiograph often shows unilateral or bilateral hazy pulmonary opacities or consolidation, predominantly in the lower zones ( Fig. 41.2 ). Bacterial pneumonia can have an identical appearance. The manifestations of pulmonary hemorrhage usually consist of bilateral ground-glass opacities or consolidation that may be patchy or diffuse ( Fig. 41.3 ).
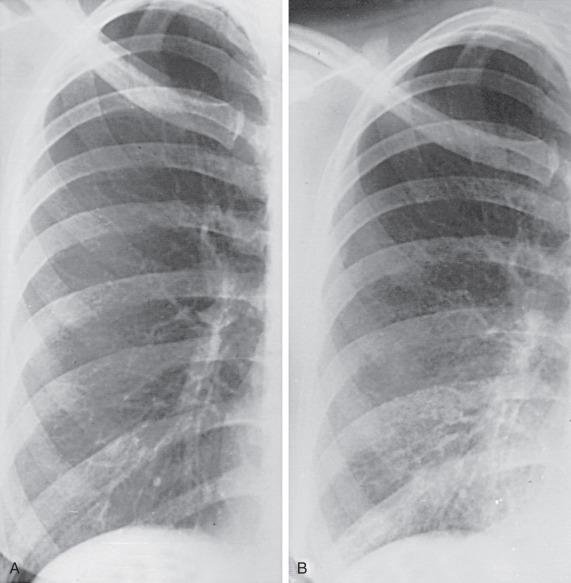
In “shrinking lung” syndrome the chest radiograph and computed tomography (CT) characteristically show elevated hemidiaphragms, adjacent atelectasis, and occasionally, small pleural effusions ( Fig. 41.4 ).
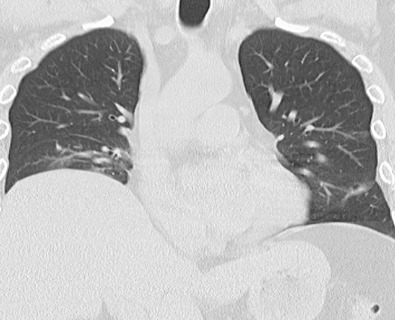
The radiographic manifestations of interstitial disease are usually mild and consist mainly of a reticular pattern in a predominantly basal distribution ( Fig. 41.5 ).
Computed Tomography
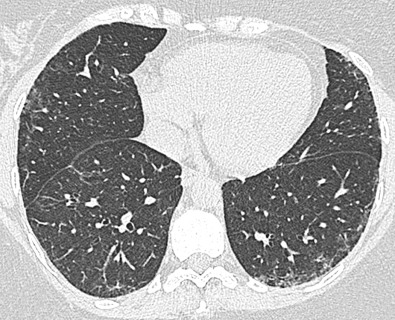
There are few high-resolution computed tomography (HRCT) studies of SLE. In a prospective series of 34 consecutive patients with SLE, 70% were found to have HRCT abnormalities. The most frequent CT findings were interstitial lung disease in a third of patients, bronchiectasis in 21%, axillary and mediastinal lymphadenopathy in 18%, and pleuropericardial abnormalities in 15%. The proportion of patients with SLE and interstitial lung disease on HRCT was higher than expected from previous studies, which, based mainly on clinical and radiographic findings, estimated that interstitial fibrosis developed in 3% to 8% of patients with SLE. The bronchiectasis seen in patients with SLE is usually mild and may be due to repeated infections or reflect an occult overlap disease.
Another CT study that focused on lung involvement in patients with SLE excluded all those with pulmonary symptoms or an abnormal chest radiograph. More than a third of patients had abnormalities on HRCT. Bronchial dilation was seen in 18% of subjects, and 13% had pleural irregularities. A third of patients had interlobular septal thickening and intralobular linear opacities, and 22% had architectural distortion. These abnormalities were located mainly in the subpleural and basal lung regions and were interpreted as representing fibrosis ( Fig. 41.6 ).