Fig. 1.1
Rapid attenuation of the radiation dose as the distance increases
The mechanism of action of this approach is described in detail later in this textbook. In brief, a study of the impact of wound fluid after BCS for breast cancer showed that wound fluid (taken from the drain over the first 24 h after BCS without TARGIT) stimulated proliferation, migration and invasion of breast cancer cell lines; however, the stimulatory effect almost completely disappeared when fluids from TARGIT-treated patients were used. This was attributed to an alteration in the molecular composition and biological activity of the wound fluid (Belletti et al. 2008). The other possible explanation is the timeliness of this approach, with delivery of radiotherapy soon after the primary tumour has been excised.
1.3.2 Historical Perspective
A Photon Radiosurgery System (PRS) was introduced at The Royal Free Hospital in 1995 to assess its role in the treatment of solitary brain metastases. The tip of the probe was placed into the tumour, using standard stereotactic techniques, allowing for the delivery of a prescribed therapeutic radiation dose directly into the centre of the metastasis. A dose of 10–20 Gy was prescribed to the margin of the treatment volume defined as the volume seen on CT scan with a 2 mm margin. Between April 1995 and April 1997 eleven patients with cerebral metastases were treated with the PRS. Ten patients were treated successfully with no morbidity and good local tumour control at the treated site at their time of death from systemic disease. One patient developed a hemiplegia postoperatively and died 35 days later from widespread systemic disease and treatment complications. This system offered the combined advantages of treatment at the same sitting as the confirmatory biopsy. On the whole it was well tolerated and provided good local tumour control (Porter et al. 1998).
Subsequently, the system was trialed for the first time in treatment of primary breast cancer at the University College London after the design and development of polymer applicators of various diameters to insert into the tumour cavity after resection (Vaidya et al. 1999).
1.3.3 TARGIT Randomised Controlled Trial
In March 2000, an international, phase 3 randomised controlled trial was launched as a non-inferiority trial and over the years enrolled patients from 33 centres in ten countries. It compared outcomes in patients aged 45 years or older with invasive ductal carcinoma undergoing BCS followed by either whole breast external beam radiotherapy (EBRT) over several weeks or a risk-adaptive approach using single-dose TARGIT. Under the risk-adaptive approach, if the final pathology report demonstrated unpredicted pre-specified adverse features, then EBRT was to be added to TARGIT afterwards, omitting boost radiation. The primary outcome measure in this study was local recurrence, with several secondary outcome measures including toxicity, survival, cosmesis, quality of life and health economics.
Randomisation to the TARGIT or the EBRT arm was done either before lumpectomy (pre-pathology) or after lumpectomy (post-pathology). Among the patients allocated to receive TARGIT, those in the pre-pathology group received it immediately after surgical excision under the same anaesthesia, while those in the post-pathology group received it as a subsequent procedure.
The original recruitment goal of 2,232 (powered to test non-inferiority; hazard ratio: <1.25) was reached in early 2010 (Vaidya et al. 2010); 1,113 patients were randomly allocated to TARGIT and 1,119 to EBRT. Fourteen percent of the TARGIT group with poor prognostic factors also received EBRT in a risk-adaptive approach, with omission of boost radiotherapy to the tumour bed, as stated above. At 4 years, there were six local recurrences in the TARGIT group and five in the EBRT group. The Kaplan-Meier estimate of local recurrence in the conserved breast at 4 years was 1.20 % (95 % CI 0.53–2.71) after TARGIT compared with 0.95 % (0.39–2.31) in the EBRT group; the difference between the groups was not statistically significant. The frequency of any complications and major toxicity was similar in the two groups and grade 3 radiation-induced toxicity was lower in the TARGIT group; however, seromas needing more than three aspirations were more common in the TARGIT group. There was no difference in wound breakdown.
The early results of this study therefore provided level 1 evidence that the single dose of radiotherapy delivered at the time of surgery using the TARGIT technique is safe and that for selected patients with early breast cancer it can be considered as an alternative to EBRT delivered over several weeks.
Recruitment to the TARGIT trial continued after the Lancet publication, primarily to allow completion of sub-protocols; the final recruitment goal of 3,451 women was achieved in June 2012 and the trial then closed to recruitment.
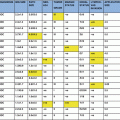
The updated results were presented at the San Antonio Breast Cancer Symposium (Vaidya et al. 2012) (Table 1.1): 1,721 patients were randomly allocated to receive TARGIT and 1,730 to receive EBRT. 1,010 patients had a minimum of 4 years of follow-up and 611 patients had a minimum of 5 years of follow-up. Primary events (local recurrence) had increased from 13 to 34 since the Lancet publication.
Table 1.1
Updated summary of the results of the TARGIT-A trial
Events | HR (95 % CI) | ||
|---|---|---|---|
5-year cumulative risk (95 % CI) | |||
TARGIT | EBRT | ||
Ipsilateral breast recurrence (IBR) | 23 | 11 | |
3.3 % (2.1–5.1) | 1.3 % (0.7–2.5) | 2.07 (1.01–4.25) | |
All recurrences (ipsilateral and contralateral breast, axillary and distant) | 69 | 48 | |
8.2 % (6.3–10.6) | 5.7 % (4.1–7.8) | 1.44 (0.99–2.08) | |
Mortality | 37 | 51 | |
3.9 % (2.7–5.8) | 5.3 % (3.9–7.3) | 0.70 (0.46–1.07) | |
For the primary outcome of ipsilateral breast recurrence (IBR), the absolute difference at 5 years was 2.0 %, which was higher with TARGIT and reached the conventional levels of statistical significance (p = 0.042), but was within the pre-specified non-inferiority margin; in the pre-pathology arm the absolute difference in 5-year IBR was 1 % and in the post-pathology arm it was higher, at 3.7 %.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree