and Alfredo Blandino1
(1)
Department of Radiological Sciences, University of Messina, Messina, Italy
Adequate intestinal distention is the main technical prerequisite for optimal small bowel imaging, as decades of experience acquired by conventional radiology with barium contrast reveal. As a collapsed bowel loop may obscure large lesions or mimic pathological wall thickening, bowel distention is the single most important factor for any method of choice. For this purpose, a large amount of enteric contrast material is used in MR examination of small bowel to achieve luminal distention. Intraluminal contrast material does not only distend the lumen, but it also decreases the susceptibility to develop artifacts by displacing intraluminal air. Actually, MREg is only a generic term, often used to define small bowel MR examination.
The proper MREg technique consists in achieving the bowel distention by oral administration of a large amount of enteric contrast agent. Conversely, when enteric contrast agent is administered through a nasoenteric tube, the term “MR enteroclysis” (MREc) should be preferred [1–3].
2.1 Enteric Contrast Agents
Various kinds of enteric contrast agents have been investigated for MREg and MREc in an attempt to achieve uniform luminal distention with minimal mucosal absorption. Other important considerations include no significant adverse effects, absence of artifacts, and low cost. The optimal contrast agent should provide adequate distention of the small bowel for the whole duration of the examination and be well tolerated by the patient.
Contrast agents can be classified into three categories, according to their signal intensity on the pulse sequences used:
(a)
Positive agents that demonstrate high signal intensity on both T1- and T2-weighted images
(b)
Negative agents that demonstrate low signal intensity on both T1 and T2 images
(c)
Biphasic agents that demonstrate different signal intensities on different sequences (Fig. 2.1) [1, 4, 5]
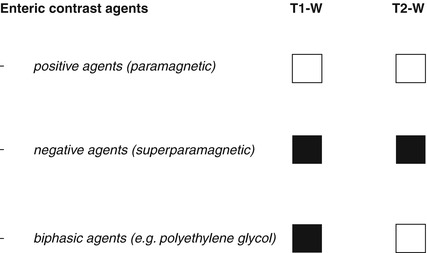

Fig. 2.1
Classification of oral contrast agents. Positive contrast agents: high signal intensity on both T1- and T2-weighted images. Negative contrast agents: low signal intensity on T1- and T2-weighted images. Biphasic contrast agents: low signal intensity on T1-weighted images and high signal intensity on T2-weighted images
Positive contrast agents are characterized by hyperintensity on both T1- and T2-weighted images. They consist of paramagnetic substances such as gadolinium chelates, ferrous ammonium citrates, manganese chloride, and food products (e.g., blueberry juice, pineapple juice) [6–8]. These contrast agents reduce T1 relaxation time, while T2 relaxation time is usually not affected. However, due to high water content of the solutions, they are also seen as hyperintense on T2-weighted images [9]. Positive contrast agents are generally disfavored because their high T1 signal interferes with evaluation of inflammatory mucosal and mural enhancement or of intraluminal lesions after i.v. contrast administration.
Negative contrast agents are characterized by hypointensity on both T1- and T2-weighted sequences. They make use of superparamagnetic particles such as iron oxides, perfluorooctyl bromide, and oral magnetic particles [3, 6, 7, 10–13]. Barium sulfate can also be used as a negative agent when administered at high concentrations, but the signal loss is not as great as with the superparamagnetic particles [9]. These contrast agents induce local dishomogeneity in the magnetic field, affecting both T1 and T2 relaxation times. They may improve the conspicuity of wall edema on T2-weighted sequences as well as the detection of extraluminal fluid collections. Due to their intraluminal loss of signal intensity, bowel wall enhancement will be more remarkable on T1-weighted images.
Superparamagnetic particles have a small incidence of side effects (5–15 % of cases) mainly related to the gastrointestinal tract (suboptimal palatability, nausea, vomiting, and rectal leakage) [5]. Moreover, if negative contrast agents are not homogeneously distributed throughout the bowel loops, they can produce paradoxical high signal intensity.
The preferences of many authors are currently directed toward the use of biphasic contrast agents and in particular for those characterized by low signal intensity on T1-weighted images and high signal intensity on T2-weighted images [3]. Following i.v. contrast administration, the low signal intensity on T1-weighted images provides a better resolution between bowel lumen and the hyperenhancing wall inflammation or masses. Moreover, these agents allow the assessment of fold thickness on T2-weighted images.
The biphasic category contains the largest number of available agents, including water, osmotic agents, non-osmotic bulking agents, and polyethylene glycol, each having unique advantages and limitations.
Although water is readily available, better accepted by the patients, and cheap, it is rapidly absorbed from distal bowel; therefore, adequate distention may not be obtained.
To obviate this problem, osmotic agents such as mannitol have been used, but these agents can cause osmotic effects in the small bowel such as diarrhea, meteorism, and abdominal cramps, which nonetheless are less significant when low-concentration solutions are used (mannitol 2.5 %) [14, 15].
Non-osmotic bulk agents such as locus bean gum and methylcellulose have been also evaluated; however, they are not widely available [15, 16].
Polyethylene glycol (PEG) solution is a hydrophilic molecule with a low partition coefficient, which determines an inconsistent transmembranous diffusion in the lipid phase. It also has a transversal diameter that does not allow it to pass through the intestinal mucosa, so it does not undergo intestinal absorption. Therefore, PEG simulates the properties of water with the advantage of non-absorbability, providing good distention of the entire small bowel. The main problem of PEG solution is that it can lead to rapid bowel transit and a strong urge to evacuate that can interfere with completion of the examination [3, 17–20].
Among the other biphasic agents, manganese and gadolinium chelates must be considered; in fact they are seen as low signal intensity on T2-weighted images and high signal on T1-weighted images when administered at high concentrations [6].
Barium products at lower weight per volume can also act as biphasic contrast agents being well tolerated by patients and providing good intestinal distention [5].
2.2 Patient’s Preparation and Positioning
Adequate patient’s preparation is mandatory because MR of the small bowel is a time-consuming technique and requires extensive hospital resources.
Patients undergoing MREg/MREc will stop ingesting solids and liquids at least 6–8 h before the examination and water up to 1–2 h prior. Instructing patients to fast before the procedure improves compliance and tolerance for ingestion of oral contrast material and ensures homogenization of bowel activity. No bowel cleansing is required.
Sedation is avoided in patients undergoing small bowel MR due to the need of patient’s collaboration for assuming enteric contrast agent.
As previously said, the use of biphasic contrast agents is preferable in MREg as well as MREc.
MREc was the first dedicated MRI method for evaluating small bowel in CD and was based on the fluoroscopic enteroclysis technique. This procedure requires that the intestinal distention is achieved via the administration of contrast material through a prepositioned nasojejunal balloon-tipped catheter [3, 5]. The placement of the catheter should be preferentially obtained under fluoroscopic guidance in a separate radiology room not to slow the work of MR suite. Balloon inflation minimizes contrast reflux back to the stomach. The instillation of contrast was originally performed in the fluoroscopic suite prior to patient transfer to MR room. Using this approach, the patient was exposed to ionizing radiations.
Nowadays, with the advent of ultrafast dynamic thick-slab (e.g., 70–180 mm) MR technique (MR fluoroscopy), contrast is now routinely instilled under “real-time” MR guidance until adequate small bowel distention is obtained. Enteric contrast material can be administered by manual injection with handheld MR-compatible infusion devices or with automated pumps, while the patient is in the MR scanner [3, 5]. The volume and the speed of infusion are crucial for the success of examination. Depending on the subject, the volume generally varies between 1,500 and 3,000 ml with an infusion rate ranging from 80 to 200 ml/min. In MREc, the contrast medium is administered in three phases:
(a)
A low infusion rate of 80–100 ml/min is used during the first phase, which lasts until terminal ileum begins to distend.
(b)
In the second phase, the infusion rate increases up to 200 ml/min to achieve reflex atony (note that if the infusion rate increases too fast, retrograde filling can often occur, and this may result in patient’s vomiting).
(c)
During the third phase, the infusion rate decreases again to 80–100 ml/min, to guarantee adequate distention of the proximal jejunum until the acquisition of cross-sectional images is performed.
MREc provides optimal distention of the bowel wall and can show detailed luminal information useful to identify early mural changes. However, these advantages have to be counterbalanced by the complexity of the procedure and by the associated patient discomfort. Moreover, an excessive distention of the bowel loops can lead to a worse assessment of the mesenteric structures, which may be compressed by nearby loops.
The MREg technique has been developed as a noninvasive alternative to MREc, due to the fact that a significant portion of patients refuses nasojejunal catheter placement. Enterography technique requires the ingestion of a large amount of fluid that fills the stomach and the small bowel in continuity.
Several different ingestion algorithms have been proposed, and they are determined by the bowel transit time of the contrast agent used and by the amount given.
In the case of PEG electrolyte solution, a total amount of 1,500–1,800 ml is generally administered within 40–60 min prior to scanning. The first 500–600 ml is ingested over the first 10–15 min and two 500–600 ml aliquots, respectively, 20–25 and 10–15 min prior to scanning, to obtain a sufficient luminal distention and to guarantee an accurate detection of lesions. Using this approach, a reasonable small bowel distention is obtained. In fact, in the majority of cases, distal small bowel is well distended in spite of proximal jejunal distention that can be variable. For this reason, some authors proposed serial acquisitions with initial imaging at 20 min after contrast medium ingestion, to assess jejunal loops in the early scans and ileum and terminal ileum in the latter scans [21].
With regard to pediatric patients, the volume of oral contrast is usually adjusted between 300 and 1,000 ml according to the weight of the subject.
Nausea and vomiting caused by oral contrast ingestion are reduced by antiemetic medication so that examination can be comfortably performed.
Although we aim for a total of 1,500–1,800 ml, some patients cannot tolerate this volume ingestion, and adequate results may be still achieved with as little as 500–600 ml. For this reason, the examination could proceed even if only a small volume of oral contrast was ingested.
Due to the poor tolerability of nasojejunal tube insertion or to excessive and uncomfortable bowel distention of MREc, in our practice we recur to enteroclysis only for the evaluation of patients unable or unwilling to ingest oral contrast for enterography.
The addition of a rectal water enema provides a better visualization of the terminal ileum and may be considered an adjunct to facilitate the evaluation of colon. However, rectal enema is not routinely performed because the colon is readily accessible at colonoscopy; anterograde filling is also possible and may be more tolerable.
To increase the signal-to-noise ratio, the patient is imaged using an abdominal phased-array radio-frequency surface coil, in either the supine or prone position (if no stoma is present).
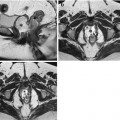
Although MRI is routinely performed in supine position for a better patient’s comfort, the preferred scanning position should be prone in order to facilitate elevation and separation of small bowel loops out of the pelvis. Moreover, this position produces a degree of abdominal compression, reducing the number of section required for each coronal acquisition, which in turn reduces the length of breath-hold required, resulting in improved patient compliance (Fig. 2.2). It has been also shown that it improves small bowel distention (Fig. 2.3) and that this position is safer if the patient should vomit.
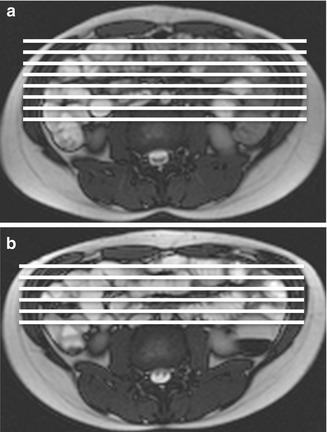
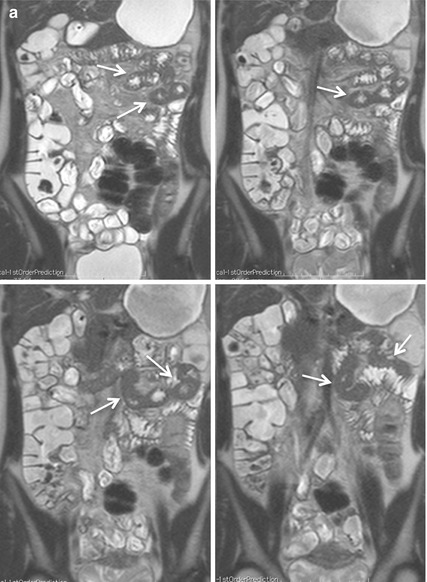

Fig. 2.2
Axial scout images obtained in supine (a) and prone position (b). Abdominal compression consequent to prone position (b) allows to reduce the anteroposterior diameter and to acquire a reduced number of coronal slices with respect to supine position (a)


Fig. 2.3
Coronal HASTE images obtained in the same patient in supine (a) and prone position (b). In supine position (a), the poor distention of jejunal and proximal ileal segments can lead to difficulties of interpretation, as the result of apparent thickening of the bowel wall (arrows). Prone imaging (b) results in significantly higher small bowel distention and better bowel loops separation, excluding any pathological wall thickening
There are no specific contraindications to MREg/MREc, except those typical for MR (e.g., metal implants in delicate positions, aneurysm clips, shrapnel injuries, pacemakers, internal defibrillators, etc.). A relative contraindication to MR examination could be considered the inability to receive gadolinium-based contrast medium (patients with a low glomerular filtration rate, who are at risk for nephrogenic systemic fibrosis or pregnant patients). However, it should be noted that in these cases the examination could be performed without i.v. contrast material, allowing one to obtain useful findings in CD.
2.3 Protocols and Sequences
In the past, motion artifacts and poor contrast resolution precluded the use of MR for bowel imaging. Technological advances, including the use of breath-hold sequences, improved coils, fat suppression, and intravenous gadolinium, have extended the role of MRI in the evaluation of gastrointestinal tract [5]. Since then, several different pulse sequences to evaluate the small bowel have been advocated by different authors. However, non-sole sequences can be used for exhaustive imaging of CD, as each sequence has its specific advantages and drawbacks: several pulse sequences may be performed and are complementary to overcome the limitations of others.
Imaging protocols vary because of differences in available equipment and personal preferences. However, despite differences in MR equipment and software, certain basic elements are common to most imaging protocols for CD [22–24]. In general, fast sequences, capable of acquiring T2-weighted and contrast-enhanced T1-weighted images in a single breath-hold reducing motion and respiratory artifacts, are the most helpful, especially with the use of biphasic contrast agent.
Once adequate small bowel distention has been obtained, the basic MREg pulse sequence protocol is substantially the same as for MREc and includes three essential sequences acquired in coronal and axial planes:
Half-Fourier acquisition single-shot turbo spin echo
Balanced steady-state free precession
Pre- and post-contrast T1-weighted ultrafast gradient echo
2.3.1 Half-Fourier Acquisition Single-Shot Turbo Spin Echo (HASTE)
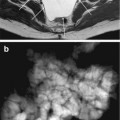
HASTE sequence is an adaptation of RARE that reduces total acquisition time by acquiring only half of k-space. Different manufactures call these sequences single–shot fast spin echo (SSFSE). These sequences provide heavily T2-weighted images in less than one second with high contrast between the lumen and the bowel wall. As these sequences are highly resistant to magnetic susceptibility or chemical shift artifacts, the wall thickness may be accurately evaluated [3, 22–24]. Moreover, sinus tract, fistulas, and collections are well visualized. HASTE sequences are susceptible to motion artifacts of fluids within the visceral lumen (propulsive intestinal movements), which may result in intraluminal low-signal-intensity artifacts and appearance of “pseudo-lesions” (Fig. 2.4) [3]. Due to k-space filtering effects, visualization of the mesenteric structures such as mesenteric vessels and lymph nodes is impaired. The optional use of fat saturation allows differentiation between submucosal fat and edema which appear both bright on T2-weighted images. Fat saturation also increases the conspicuousness of edematous bowel loops and the contrast between the intestinal wall and the surrounding fat tissue. Fat-suppressed HASTE sequences are particularly useful when i.v. contrast material administration is contraindicated to assess parietal inflammatory infiltration and to reveal inflammation of the peritoneal fat tissue [3]. Moreover, the use of fat saturation allows the acquisition of thicker sections to demonstrate all bowel loops on a single image (Fig. 2.5).
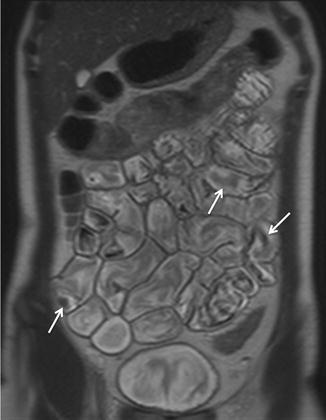

Fig. 2.4
Coronal HASTE image. Normal small bowel. Intraluminal flow voids, secondary to bowel peristalsis, are seen (arrows)

Fig. 2.5
Coronal thick-slab HASTE image (50-mm thickness) obtained with fat saturation showing several bowel loops in a single image
2.3.2 Balanced Steady-State Free Precession (Balanced SSFP)
Another sequence promoted for imaging of the small bowel is the balanced SSFP. Other vendors call these sequences fast imaging with steady–state acquisition (FIESTA), balanced fast field echo (balanced FFE), and true fast imaging with steady-state precession (TrueFISP).
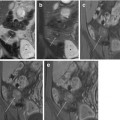
This sequence consists of a balanced gradient-echo sequence where image contrast is dependent on T2/T1 ratio for each tissue. The ratio of T2/T1 contrast essentially reflects the T2 differences in tissues as repetition time and echo time are so short that T1 is almost constant: tissues with a T2 value approaching their T1 value appear brightest. Moreover, these sequences eliminate phase shift caused by motion, and thus both fluid and blood appear bright. Due to the extremely short acquisition time, with each image acquired in a few hundred milliseconds, they are relatively insensitive to motion artifacts, providing uniform intraluminal signal and leading to a high contrast between the bowel wall, lumen, and mesentery (Fig. 2.6). In particular, mesenteric vessels and adenopathies are better visualized on these sequences than on the HASTE sequences.
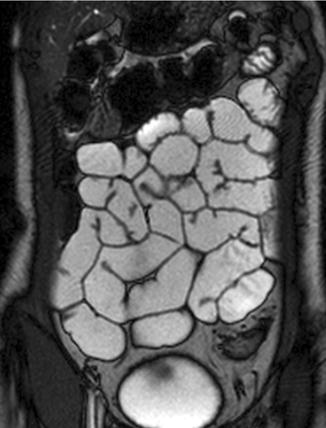

Fig. 2.6
Coronal TrueFISP image. Normal small bowel with uniform intraluminal signal and high contrast between bowel wall and lumen. The “black-boundary” artifact may not be confused with wall thickening
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree