9 The head, neck and spine
ULTRASOUND TECHNIQUE
The availability of Doppler is extremely useful for the routine cranial examination. This is particularly true when trying to differentiate subdural from subarachnoid fluid in the subarachnoid spaces and in any suspected vascular lesion such as a vein of Galen anomaly. It has also been used in assessing vascular malformations post-embolization.1 Doppler ultrasound has been used to monitor the neonatal brain, in particular birth asphyxia, brain injury, hydrocephalus and brain death, with varying results. Transcranial Doppler in children with a closed fontanelle has been used in a limited way. They have been reportedly used in children with sickle cell anemia and to determine brain death.2
Measurements
Ventricles
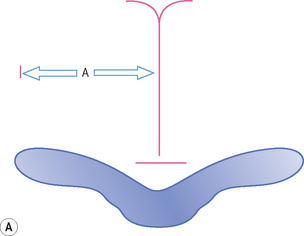
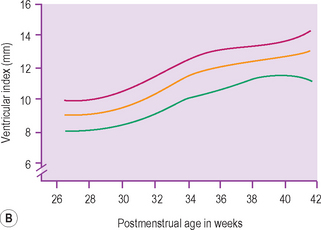
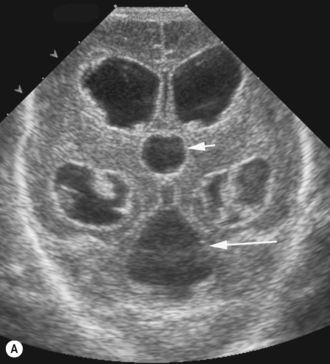
There is a large range of ventricular size, both prenatally and in the newborn. An integral part of the cranial ultrasound examination, in particular when ventricles are dilated, is a measure of the degree of dilation. There are many coronal and sagittal measurements quoted in the literature and these are just a confusing array for the uninitiated. It is important to have a standard technique and measurement for a department so that it is consistently reproducible between sonographers undertaking the examinations and so that the clinicians clearly understand the measurements produced. There is still no consensus as to which is best but the most universally accepted measure is the ventricular index as described by Levene. This chart is for preterm infants. The index measures the distance from the falx to the lateral border of the lateral ventricle. This is measured coronally in the plane of the third ventricle at the level of the foramen of Munro. The ‘ventricular index’ is measured as the largest distance between the frontal horns. Dividing this number by 2 results in the ‘ventricular index’. The measure is easy to understand and is reproducible. Clear visualization of the lateral ventricles can be obtained and landmarks clearly identified. It shows little interobserver error. The centile chart expressing the 3rd, 50th and 97th centiles from 26 to 42 weeks has been produced. The only problem with Levene’s ventricular index is when there is midline shift (Fig. 9.1). Measurements of ventricular size in term babies are shown as well.3 The author has also published similar charts for preterm babies.
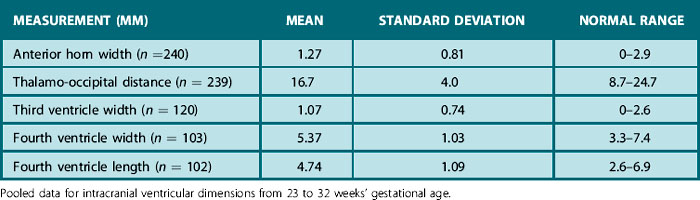
According to Davies4 the ‘anterior horn width’ is the width of the anterior horn of the lateral ventricles measured in the 3D coronal view at the level of the foramen of Munro. The width is measured on each side as the distance between the medial wall and floor of the lateral ventricle at the widest point. The linear measurements taken in their study of infants with a mean age of < 33 weeks’ gestational age at birth were
• thalamo-occipital distance of the lateral ventricles
• width of the third ventricle
• width and length of the fourth ventricle (Fig. 9.2 and reference values Table 9.1).
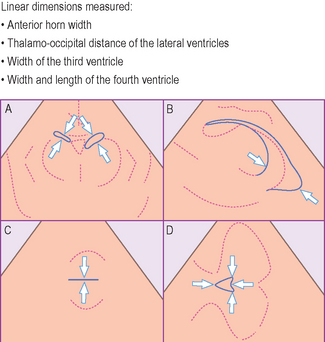
Figure 9.2 A line drawing showing the measurements made on the ultrasound images, with arrows indicating the correct caliper position and alignment (A, coronal plane; B, parasagittal plane; C & D, transverse plane). Reference values are given in Table 9.1.
The occipital poles enlarge before the body of the lateral ventricles, but measurements are unreliable and impractical due to the wide range of size and shape of the occipital horns. Measuring the biventricular diameter is also not reliable, as one ventricle may enlarge more rapidly than another (Box 9.1).
BOX 9.1 Tips for cranial ultrasound
• If monitoring equipment in situ, ensure it is well secured
• Get parent or helper to immobilize the head
• Use a high-frequency transducer that fits snugly onto the anterior fontanelle. Try supplementary views if needed
• Start in the coronal plane and look for symmetry of the right and left cerebral hemispheres and intracranial structures
• Check that the normal anatomic structures are distinguishable
• Does the cortical folding appear appropriate for age?
• Is there normal distinction between cortex and white matter?
• Is there appropriate echogenicity of the periventricular and subcortical white matter and the basal ganglia and thalami?
• Is there peri- or intraventricular hemorrhage?
• Asymmetric, echogenic (greater than the choroid plexus) areas are usually abnormal
• Occipital flare can be a normal finding
• Evaluate the subarachnoid space and fissures: > 5 mm is usually abnormal
• Evaluate the size and width of the ventricles. Asymmetry in ventricular size can be normal
• Slit-like ventricles in the absence of other changes can be normal
Subarachnoid space
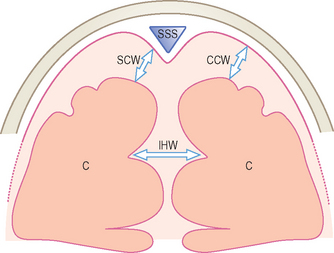
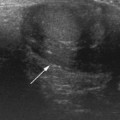
The subarachnoid spaces should be carefully evaluated and linear dimensions taken in the coronal plane at the level of the foramen of Monro (Fig. 9.3).5
• Craniocortical width (CCW) — widest vertical distance between calvarium
• Sinocortical width (SCW) — widest distance between lateral wall of superior sagittal sinus and surface of cortex
• Interhemispheric width (IHW) — widest horizontal distance between the hemispheres
Normal anatomy
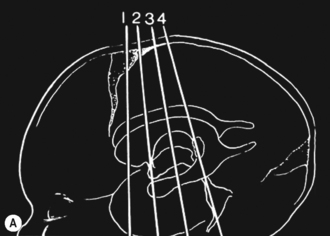
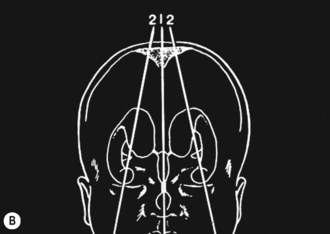
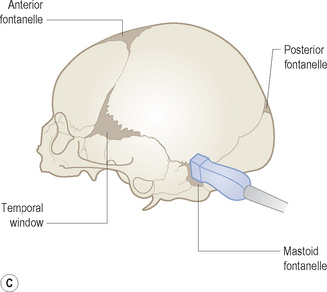
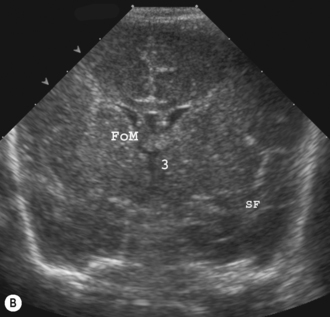
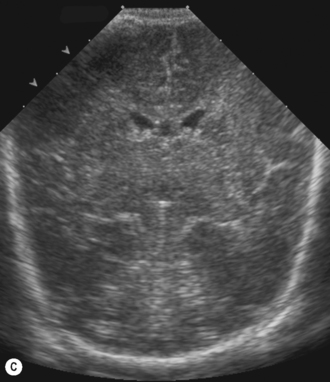
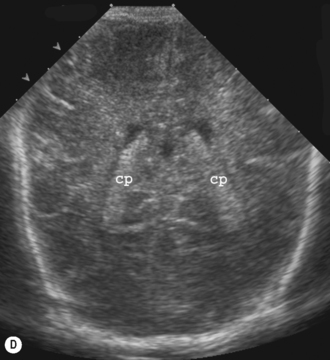
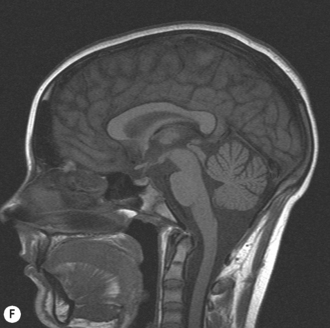
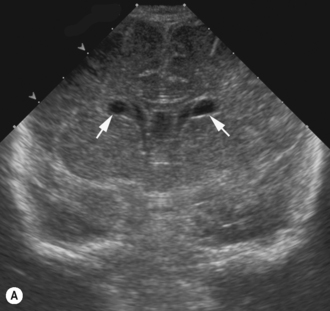
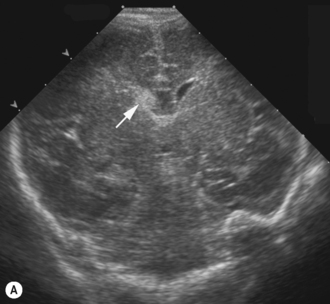
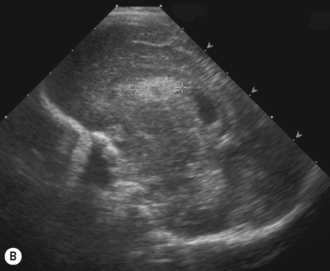
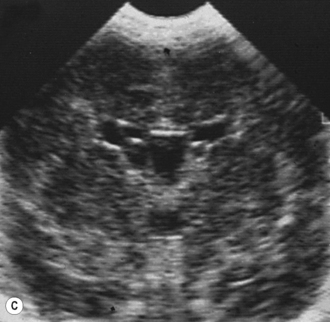
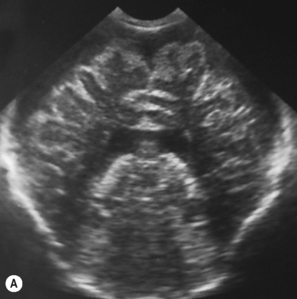
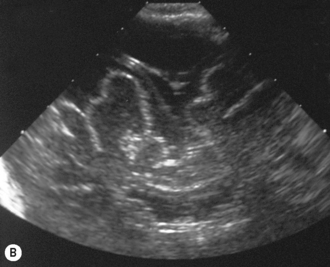
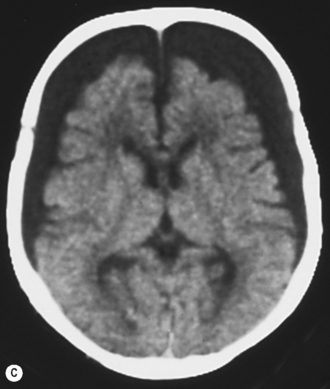
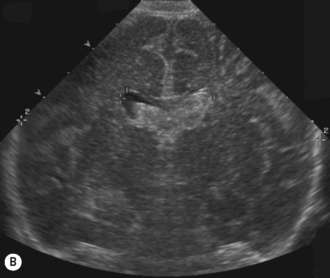
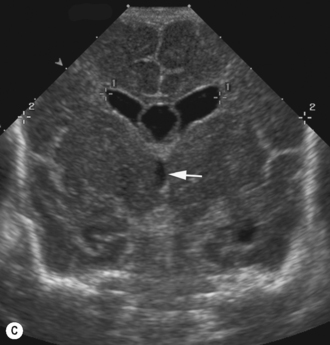
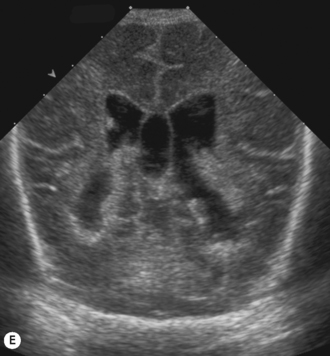
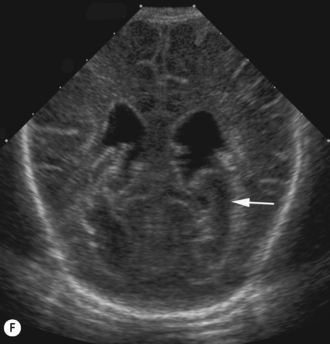
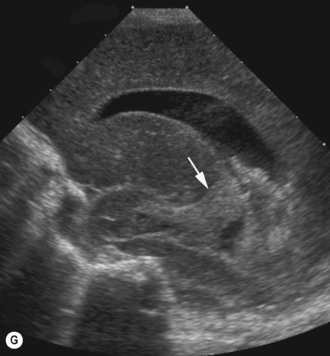
Cranial ultrasound examinations are performed in the coronal and sagittal planes (Fig. 9.4A,B). Additional acoustic windows are found through the temporal bone and mastoid fontanelle (Fig. 9.4C).
Axial planes
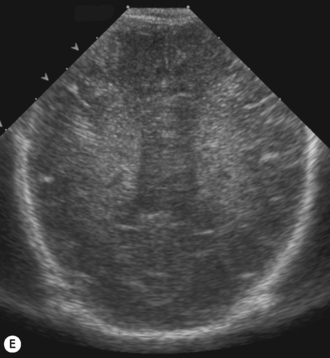
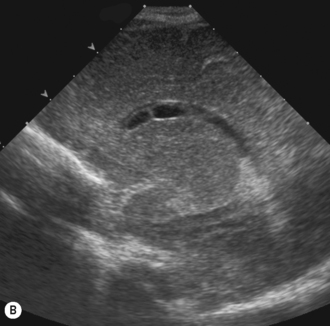
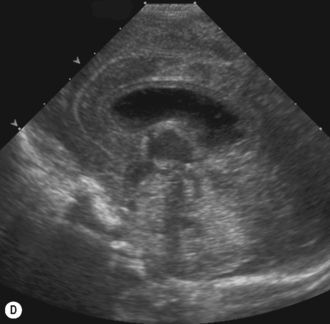
Axial planes are not routinely used but can be extremely useful if subdural or extra-axial fluid is suspected. Axial sections can be difficult to understand, so are best taken at the body of the lateral ventricles where anatomy is easily recognized (Fig. 9.5).
The ventricular system
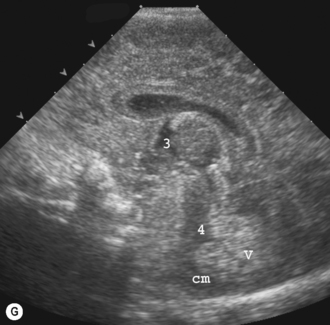
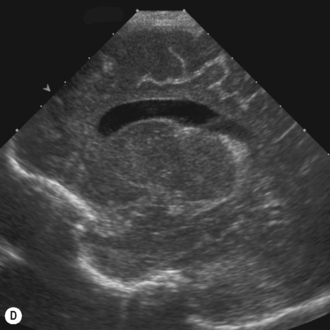
The lateral ventricles are the largest and consist of the frontal horn, the body, the temporal horn and the occipital horn. They all communicate and, in addition, they communicate with the subarachnoid spaces. They communicate with the third ventricle via the foramen of Munro and the fourth ventricle via the aqueduct of Sylvius. The third ventricle is not easy to see on the coronal scan as it is a slit-like structure, neither is the aqueduct on the sagittal scan. The fourth ventricle has the typical rhomboid ‘Napoleon’s hat shape’ and extends into the cerebellar vermis (Fig. 9.6).
Coarctation of the lateral ventricles is a normal variant, where there are cystic areas adjacent to the superolateral angle of the lateral ventricle at the level of the foramen of Munro. These cysts appear as a string of beads along the floor of the lateral ventricle. In some of the literature this is called subependymal pseudocysts. It is important not to confuse these with periventricular leukomalacia (Fig. 9.7).6
The basal ganglia
The basal ganglia is the area seen best at the level of the foramen of Munro and consists of the caudate nucleus and thalamus. They are separated by the caudothalamic groove, an important landmark when assessing for germinal matrix hemorrhages. The two thalami are connected via the massa intermedia, which can be seen in a pathologically dilated third ventricle (see Fig. 9.6).7
INTRACRANIAL HEMORRHAGE
All infants under 34 weeks should be routinely scanned.
The following should always be assessed:
• basal ganglia, particularly the area of the germinal matrix in the floor of the lateral ventricles
• ventricles for size and intraventricular hemorrhage
• cerebral parenchyma, particularly periventricular region for flares
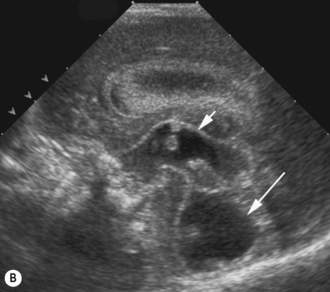
• extra-axial fluid such as subdural and subarachnoid collections (Fig. 9.8).
Types of intracranial hemorrhage
Germinal matrix
This is a small area of brain occurring in the floors of the lateral ventricles and best seen on anterior coronal scans. This area is very vascular in the premature infant and that is why these hemorrhages occur primarily in the premature infant. It has a rich matrix of fragile capillaries and it is alterations in cerebral blood flow that causes vasodilation of these tiny vessels leading to hemorrhage. This may be an isolated finding but they occasionally will rupture into the ventricle and in a small percentage will rupture into the parenchyma through the roof of the lateral ventricle. By term and increasing gestational age the subependymal plate has almost completely disappeared. There is almost universally a good prognosis, with only a very small number (2%) reportedly resulting in neurological sequelae.8
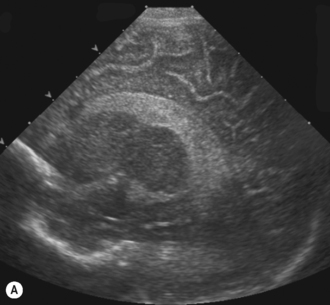
Small germinal matrix hemorrhages, if they are fresh, appear echogenic. They may occasionally be mistaken for choroid in the floor of the lateral ventricle. Hemorrhages need to be confirmed in the sagittal view and occur anterior to the caudothalamic groove. There is no distortion of the ventricular system and after a number of weeks they may become cystic and eventually disappear as the brain grows (Fig. 9.9).
Intracerebellar hemorrhage
Cerebellar hemorrhage is well described and may occur in the preterm and term infant. Hemorrhage may occur due to a bleeding disorder or trauma, and has been associated with tight-fitting face masks.9 The cerebellum is normally echogenic but hemorrhage may increase the echogenicity. It may be unilateral with an irregular outline.
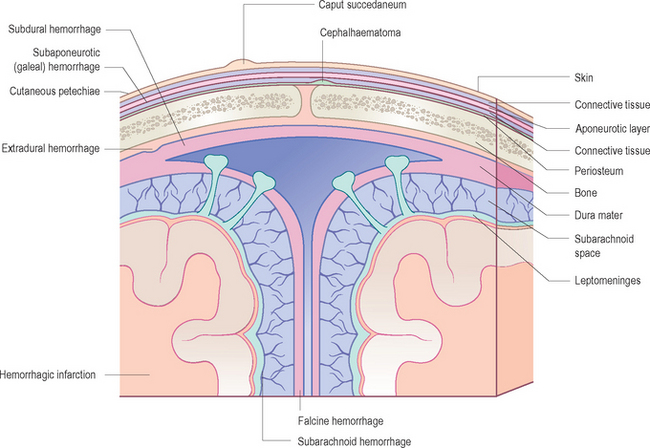
Subdural hemorrhages
Subdural hemorrhages are caused by trauma at birth, non-accidental injury, and bleeding disorders. Bleeding results from dural tears.10–12
Classification
There is no internationally accepted grading system for germinal matrix hemorrhage/intraventricular hemorrhage (GMH–IVH). Tables 9.3 and 9.4 outline the grading systems for periventricular hemorrhage used for ultrasound scanning.
Table 9.3 Grading system for PVH used or adapted for use with ultrasound brain scanning9
| Grade I | Isolated SEH |
| Grade II | Rupture into ventricle, but no dilation |
| Grade III | Rupture into ventricle with dilation |
| Grade IV | IVH with parenchymal extension |
IVH, intraventricular hemorrhage; SEH, subependymal hemorrhage.
Table 9.4 A modification of the grading system suggested by Levene and de Crespigny14
| HEMORRHAGE | |
| 0 | No hemorrhage |
| 1 | Localized hemorrhage <1 cm in its largest measurement |
| 2 | Hemorrhage >1 cm in its largest measurement but not extending beyond the atrium of the lateral ventricle |
| 3 | Blood clot forming a cast of the lateral ventricle and extending beyond the atrium |
| 4 | Intraparenchymal hemorrhage |
| VENTRICULAR DILATION | |
| 0 | No dilation |
| 1 | Transient dilation |
| 2 | Persistent but stable dilation |
| 3 | Progressive ventricular dilation requiring treatment |
| 4 | Persistent asymmetrical ventricular dilation |
The advantage of Papille’s grading system is that it is simple and easy to understand.9 Levene’s grading system has also gained some acceptance but it is more complicated and neither take account of periventricular leukomalacia.13
It is far better to document the site and size of the hemorrhage and, if there is ventricular dilation, to measure it (Fig. 9.11).