6 The knee
Introduction
Before magnetic resonance (MR) imaging became readily available in the 1990s, comprehensive imaging of the frequently injured internal structures of the knee joint was primarily accomplished with arthroscopy. In comparison to MR imaging, this procedure is both invasive and expensive and, in modern clinical practice, arthroscopies are no longer routinely performed; they do, however, still have a role in clarifying inconclusive findings and, of course, for therapeutic benefits. Magnetic resonance is now considered the gold standard for intra-articular imaging of the knee1,2; indeed, the knee is the joint most commonly imaged using this modality, partly because the joint is particularly vulnerable to both acute and chronic injury and partly because of the accuracy and detail afforded to both the surgeon and the physician.3,4
Since the first successful nuclear resonance experiment on biological tissue in 1946,5 there have been tremendous improvements in equipment, computer software, protocols and clinical interpretation. These advances have helped to improve image quality and diagnostic accuracy (including the appearance of normal variants) and reduce the effects of imaging artifacts.
History and examination
Perhaps more than any other joint, the knee has an embarrassment of recognized orthopaedic tests, usually eponymous and often of unproven sensitivity and reliability6,7; however, a standard examination routine need contain no more than a handful of such tests if the history and examination are properly structured.
As with any conscious and articulate patient, obtaining a full and competent history should form the backbone of the clinician’s diagnostic work-up. There are several factors that are of particular importance in the knee; these are detailed in Box 6.01 and should be capable of guiding the physician to the most likely involved structures, informing their differential diagnosis and guiding their physical examination.8,9
This should commence not with the knee itself but with general observation of the patient and their height, weight and somatotype, with calculation of the body mass index (BMI) if necessary; more than in any other joint, the incidence of degenerative change in the knee is linked to obesity.10–14 Evaluation of gait can give a good indication of the severity of the injury and the degree of disability, and assessment of stance can direct the clinician to asymmetries of alignment; genu varum or genu valgum; and tibial torsion.9 Pes planus, which can significantly increase valgus knee stress, can also be readily identified at this stage. This observation can be extended to the local area about the knee, wherein any discoloration, swelling, atrophy or displacement should be noted and investigated further.15
This should be augmented by palpation, which should assess any areas of abnormal temperature and the quality of any swelling as well as attempting to elicit specific site tenderness and identify trigger points; in particular, the hypertonic popliteus muscle is a commonly overlooked cause of pain and perpetuation of knee pathomechanics.16 The femoral, popliteal and dorsalis pedis pulses should all be checked and compared. Many conditions, such as vascular disease, bursitis, tendonitis, ligamentous sprain, patellar subluxation, Baker’s cyst, neuroma and some osteochondral defects, can be identified, if not positively confirmed, by history, observation and expert palpation, and local joint line tenderness has been shown to be a sensitive and reliable test for determining a meniscal lesion.17
In the younger patient, where symptoms allow, there is a range of functional tests that can be performed; these are particularly useful in assessment of the high-performance athlete with persistent yet subtle lesions and of knee laxity or instability9 and are detailed in Table 6.01. In the less able patient, active and passive ranges of motion will suffice: flexion, extension plus valgus and varus stress, which can best assess collateral ligament injury; these should be compared to the unaffected side. Movement of the knee may also elicit clicking, popping or grating, suggestive of a foreign body or meniscal tear; this can be further assessed using McMurray’s test, which consists of flexing and extending the knee in neutral, then with internal and external tibial rotation whilst the thumb and fingers are placed over the joint line.
Table 6.01 Functional knee tests
| Test | Ask the patient to… |
|---|---|
| Stationary jog | Jog gently on the spot |
| Fast jog | Increase the tempo and increase their knee lift |
| ‘Housemaid’s knee’ | Kneel upright |
| ‘Parson’s knee’ | Kneel whilst leaning backwards |
| ‘Duck waddle’ | ‘Walk’ whilst fully squatting |
| Hopping | Hop on the affected limb with the toe pointing inwards and then outwards |
Special tests are also needed to assess the cruciate ligaments. The more commonly injured anterior cruciate ligament is evaluated using the modified Lachman’s test whereby the supine patient lies with their hip and knee flexed to approximately 20° and the examiner, thumbs on the proximal tibia, draws the distal extremity anteriorly; instability is noted if the excursion is greater than on the unaffected side. The posterior drawer test is used to assess the posterior cruciate ligament and involves the same position for both patient and examiner but this time the tibia is drawn posteriorly.9
Although there is a plethora of special tests and measurements for assessment of the patella in the patient with patellofemoral pain syndrome, many of these are of dubious or, at best, unproven value.18 A competent assessment of the patella consists mainly of observation of the structure both statically and dynamically. Conditions involving frank abnormalities of the patella such as luxation, patella alta, patella baja or agenesis are usually immediately obvious19; palpation can also readily determine bipartite patellae, the absence of pain or previous trauma will usually be sufficient to differentiate this from fracture or non-union.
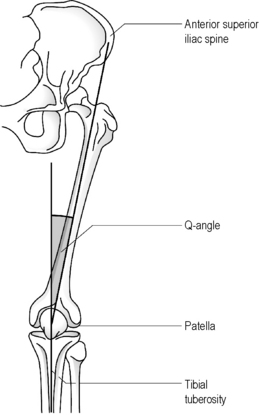
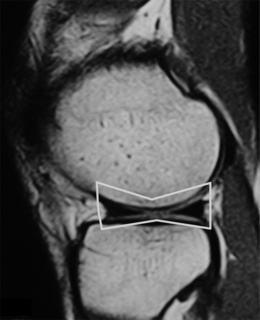
Although radiographs may be required to precisely determine the quadriceps angle or Q-angle (Figure 6.01), the experienced practitioner should be able to determine when this falls outside the normal range of 10–15° for men and 15–23° for women9,20,21; an increased Q-angle is strongly linked to patellofemoral pain.20
Assessment of muscle bulk can also be a key indicator of patellar tracking syndrome. Unless bilateral MR images have been acquired, this assessment will not generally be aided by specialist imaging. Although protocols have been developed for kinematic MR imaging, enabling the patella motion to be captured during early flexion, the expense involved coupled with technical difficulties in reliably acquiring the sequences have severely restricted the application of MR imaging to patellar tracking assessment.18,22
The diagnosis can best be checked by the squat test; in healthy subjects the patella will rotate medially at 90° of knee flexion, whilst in patient with abnormal patellar function the rotation will be lateral.23 During the squat, it is also possible to ascertain and, with practice, source any crepitus or clunking which may be associated with patellar subluxation or degeneration or with intra-articular pathology.9,15
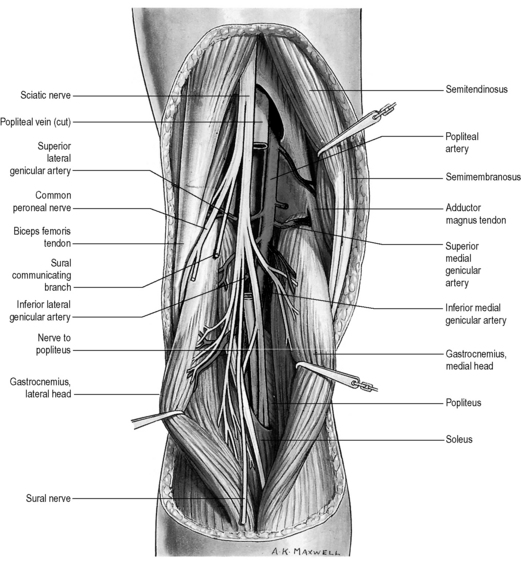
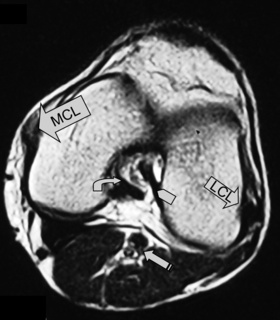
Finally, any assessment of the knee articulation should include a thorough neurological assessment24; not only can lumbar radiculitis refer pain to the knee, it can also disrupt the functionality of the major muscle groups attaching about the joint. Local nerve damage also needs to be eliminated; the peroneal nerve is vulnerable as it passes superficially over the head of the fibula and the popliteal fossa houses the tibial, common peroneal, sural and posterior femoral cutaneous nerves as well as an articular branch from the obturator nerve (Figure 6.02).25–27
Differential diagnosis
Several challenges face the clinician seeking to establish and refine a differential diagnosis for the patient with knee pain. Although the history can give strong clues from the mechanism of injury, the onset of pain can often be insidious or inconclusive: the symptoms can often be due to dysfunction elsewhere in the kinematic chain either from biomechanical consequence or by referred pain. This latter is particularly crucial when considering paediatric cases; both slipped capital femoral epiphysis and Legg–Calvé–Perthes disease can present as knee pain with no hip symptoms – and the cost of a missed diagnosis can be serious for the patient. The age of the patient should help to direct the clinical thinking.28–30
Knee pain can also be a symptom of systemic disease: crystal disease, or inflammatory or septic arthropathy. A list of common conditions causing knee pain is given in Table 6.02.28,31 As well as the history, the location of the knee pain should also help to guide and clarify the clinician’s thinking; a list of conditions that can be suggested by location alone is given in Box 6.02.28
Table 6.02 Differential diagnosis of knee pain by age
| Children and adolescents | Adults | Older adults |
|---|---|---|
| Patellofemoral pain syndrome | Patellofemoral pain syndrome | Osteoarthritis |
| Osgood–Schlatter disease | Medial plica syndrome | Crystal-induced inflammatory arthropathy: gout, calcium pyrophosphate crystal deposition disease (pseudogout) |
| Jumper’s knee (patellar tendonitis) | Pes anserine bursitis | Popliteal cyst (Baker’s cyst) |
| Referred pain: slipped capital femoral epiphysis, Legg–Calvé–Perthes disease | Trauma | Neoplasm: metastatic cancer |
| Osteochondral defect | Inflammatory arthropathy: rheumatoid arthritis, Reiter’s syndrome (reactive arthritis), pigmented villonodular synovitis | Trauma |
| Juvenile rheumatoid arthritis (Still’s disease) | Tendonitis/bursitis | Synoviochondrometaplasia |
| Trauma | Septic arthritis | |
| Neoplasm: Ewing’s sarcoma, osteosarcoma | Stress fracture/Stress reaction | |
| Referred pain: neurogenic, hip and leg pathology | ||
| Neoplasm: osteochondroma, aneurysmal bone cyst |
Protocols
Most imaging protocols for the knee include sagittal, axial and coronal images with the field of view ranging from 14 cm to 16 cm, depending on patient size. The slice thickness usually varies from 3 mm to 5 mm with an interstitial gap of about 0.5 mm. Gaps, or areas of non-imaged anatomy in between the slices, are used to decrease ‘cross-talk’ in two-dimensional imaging acquisition;32–35 cross-talk is the presence of unwanted signals coming from adjacent anatomy.
Normal anatomy and common variants
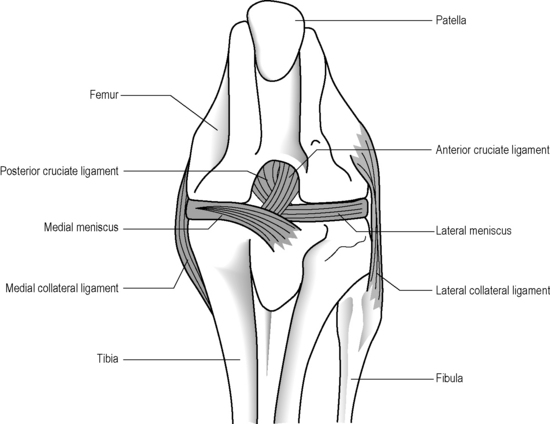
The knee is often described as a synovial hinge joint;27,36,37 however, this is a gross simplification from both an anatomical and biomechanical perspective; the femorotibial joint is the largest in the body and also one of the most complicated (Figure 6.03). The presence of the menisci make the joint complex and, of course, the knee consists of more than just this single joint; the proximal tibiofibular joint, a synovial plane joint, and the patellofemoral articulation make the knee, functionally, a compound joint, most correctly defined as a bicondylar joint.25,38,39 Unlike a true hinge joint, the knee demonstrates limited rotation about two other orthogonal axes, it can increase its valgus and varus angles passively and is reliant on femoral rotation to obtain its highly stable close packed position.38,39
In general, the menisci can be evaluated using single echo proton density images. Spin echo T1-weighted images and gradient echo techniques can also be very useful. When assessing ligaments, tendons or other soft tissues, the preferred techniques will be fluid-sensitive, or T2-weighted, sequences. Hyaline cartilage and loose bodies can be best evaluated using gradient echo techniques, with or without fat suppression, and fat-suppressed spin echo sequences.32–34
Menisci
The menisci of the knee are well visualized and commonly evaluated with MR imaging. The normal meniscus is a semicircle of cartilage and collagen fibres, thicker peripherally and thinner centrally; they display low signal intensity on all sequences (Figure 6.04).
The medial meniscus is slightly bigger and has a larger radius than its lateral counterpart. The ‘horns’ of the latter are usually of equal size; in the medial meniscus, the anterior horn is larger than the posterior.3,25,27
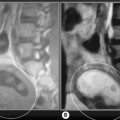
On the sagittal slices, each meniscus has a ‘bow-tie’ appearance (Figures 6.05, 6.06). Towards the midline, the menisci have the appearance of two triangles.32–34,40 In some individuals, the morphology of the meniscus is more representative of a disc. ‘Discoid’ menisci are variable in appearance; many are not true discs but merely have wider-than-normal bodies.41,42 The incidence of discoid meniscus is estimated at being between 3% and 4.5%; they are nearly always found in the lateral meniscus.43–45 On the sagittal views, the meniscus will not demonstrate the typical ‘bow-tie’ appearance; the pathological implication of this morphological variant will be discussed later in the chapter.
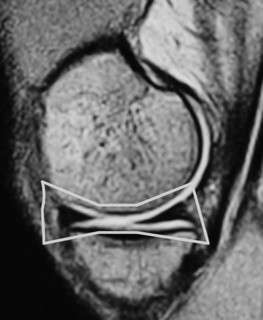
Figure 6.06 • Sagittal T1-weighted MR image of the right knee of a 27-year-old male at the level of the medial compartment demonstrates the ‘bow-tie’ appearance of the medial meniscus. Notice the wider configuration of the medial meniscus compared with that of the lateral meniscus noted in Figure 6.05.
Intrasubstance signal may be present on the peripheral edge, representing vascularity; this finding is more prominent in younger patients and may be mistaken for a tear. The popliteus tendon adjacent to the lateral meniscus and the normal attachments of the meniscofemoral ligaments on both the medial and lateral side are other structures occasionally mimicking tears.32–35
A common normal variant that can mimic pathology is a ‘speckled’ anterior horn lateral meniscus. Present in as many as 60% of normal patients, this appearance is due to fibres of the anterior cruciate ligament inserting into the meniscus; it can present an appearance similar to that of a macerated anterior horn.46
Ligaments
Cruciate ligaments
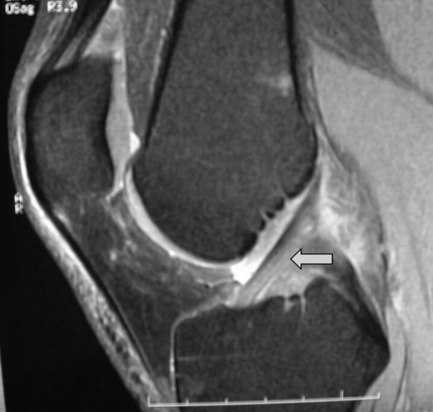
The anterior and posterior cruciate ligaments are two other structures very commonly evaluated with MR imaging. Both ligaments are intra-articular but extrasynovial structures linking the femur and tibia (Figure 6.07).25,36
The anterior cruciate ligament (ACL), which is frequently injured during athletic activities, is a very important structure providing stability to the knee and preventing anterior translation of the tibia. It also prevents excessive internal rotation and hyperextension. The ACL attaches at the posterior aspect of the lateral femoral condyles and on the medial anterior aspect of the tibial plateau. It does not, contrary to popular belief, insert on the tibial spines.25,27 The ACL is best visualized on sagittal images (Figures 6.08, 6.09). On some scans, the small anteromedial and larger posterolateral fibre bundles can be differentiated, especially if the patient’s lower extremity is placed with approximately 5° of external rotation in order to align the fibres with the plane of the scan; this can give the structure a striated appearance.3 On the coronal slices, the ACL appears as a flattened structure adjacent to the lateral femoral condyles. There can often be some high signal within the ligament, most particularly near its insertion on the tibia.2