▪
Pleural Anatomy
The pleura is a stroma supported by the mesothelial lining of the thoracic cavity. It is composed of two layers: an inner visceral pleura and an outer parietal pleura. The visceral pleura lines the lungs and their fissures. The visceral pleura is supplied by pulmonary arterioles, with drainage via the pulmonary veins. The parietal pleura lines the chest wall, diaphragm, and mediastinum. The parietal pleura is supplied by the systemic circulation and drains via the azygos, hemiazygos, and internal mammary veins.
Pleural Space and Its Normal Contents
The pleural space is the potential space between the visceral and parietal pleura. The right and left pleural spaces do not communicate. Although the pleura is permeable to gas and liquid, there is no gas within the pleural space under normal conditions. In contrast, the pleural space contains approximately 5 mL of physiologic pleural fluid, which acts as a lubricant. Pleural fluid is produced at a rate of up to 100 mL of fluid/h, and the pleural space can absorb fluid at a rate of approximately 300 mL/h. Lymphatic drainage of the pleural space, which allows for clearance of cells, protein, and other particulate matter, occurs through the stoma in the parietal pleura, which leads to lymphatic vessels in the chest wall and ultimately the thoracic duct. The lymphatic drainage of the visceral pleura occurs via the lymphatics in the pulmonary interstitium and drains into hilar nodes.
Imaging the Pleura: Indications and Advantages of Different Modalities
Evaluation of Pleura
The pleura and pleural space can be directly visualized and, if required, sampled by video-assisted thoracic surgery or open surgery. These procedures are invasive and can lead to complications. Imaging allows noninvasive evaluation of the pleura and is useful for diagnosis, procedural planning, and, if required, image-guided intervention.
Indications for Plain Radiography
Plain radiography is useful as a low-cost screening evaluation of the pleura and pleural space and can detect some different pathologies, including pneumothorax, effusion, and pleural masses, although characterization of abnormality is limited due to poor contrast resolution. The margins of the pleura are delineated by the interface of air in the lungs and soft tissue density along the chest wall, mediastinum, and diaphragm. Pleural abnormalities can be suspected when this interface is obscured or when abnormal soft tissue contours are seen along the chest wall or mediastinum. For example, dependent pleural fluid collects in characteristic locations on upright (costophrenic angles) and decubitus radiography, although loculated fluid may have a fixed masslike shape ( Fig. 35.1 ). Plain radiography is also useful in following known pleural pathology, such as monitoring pleural effusion or pneumothorax size.

Indications for Ultrasound
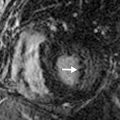
Ultrasound differentiates between fluid and solid tissue and can be used as a troubleshooting tool in the further evaluation of radiographic abnormalities. Sonography is particularly suited for the evaluation of pleural effusion and image-guided thoracentesis. Effusions appear as anechoic collections, which are easily distinguished from the echogenic ribs and dirty shadowing from the air-containing lungs. Echogenic septations may be seen on ultrasound, which are not evident on other imaging modalities ( Fig. 35.2 ). In patients who have failed blind thoracentesis, sonography is useful for localizing loculated collections of fluid. Ultrasound guidance reduces the risk of complication from thoracentesis. Also, ultrasound can be performed at the bedside, allowing assessment of critically ill patients who may not be stable enough to travel to the radiology department.

Indications for Computed Tomography
CT provides superior spatial resolution and excellent contrast resolution in the evaluation of thoracic structures, including the pleura, particularly when IV contrast is administered. The major strengths of CT include not only the detection of an abnormality, but also compositional analysis and precise localization (pleural, extrapleural, or parenchymal). CT is the most sensitive modality for the detection of pleural effusion and pneumothorax and is also sensitive for the detection of pleural masses, pleural thickening, and calcification. In contrast to plain radiography, CT allows cross-sectional evaluation of the entire pleural space, as well as adjacent thoracic structures. Multiplanar reconstruction can be achieved with a single sequence of image acquisition. IV contrast can help differentiate solid from cystic lesions and an empyema from a lung abscess. CT is the modality of choice in the routine evaluation of pleural masses, pleural malignancy, degree and extent of pleural thickening, and asbestos-related pleural disease.
Indications for Magnetic Resonance Imaging
The role of routine MRI of the pleura is limited. Although MRI has superior soft tissue contrast in comparison with CT, the spatial resolution, acquisition time, and cost of MRI prohibits its routine use in the evaluation of pleural disease. MRI is a useful adjunct to CT in the evaluation of pleural masses and malignancy because it is more accurate and sensitive than other modalities in the detection of the chest wall, mediastinal, and diaphragmatic invasion. Lung and pleural pathology in the apices are often better visualized on MRI than on CT. Some studies have suggested that MRI can also be helpful in elucidating the cause of pleural effusion. However, the MRI signal characteristics are usually nonspecific.
Indications for Positron Emission Tomography
Positron emission tomography (PET) with fluorodeoxyglucose (FDG) can be a useful problem-solving tool in the evaluation of certain pleural diseases, especially in combination with diagnostic quality CT. PET-CT is more accurate than CT, MRI, or PET alone for the staging of malignancy (including malignant mesothelioma), is superior in the detection of intrathoracic and extrathoracic lymphadenopathy, and can be used to assess response to treatment. It can also be used to differentiate malignant mesothelioma and pleural metastasis from most benign pleural diseases. Malignancy usually demonstrates higher FDG avidity, although infection or talc pleurodesis can also be intensely hypermetabolic.
▪
Pleural Effusion
Importance of the Size of Pleural Effusion
Although size does not necessarily correlate with the patient’s symptoms, it does affect the perceived severity of disease, and larger pleural effusions are more likely to be symptomatic. Effusion size is also a predictor of ease of thoracentesis and can help assess the need for image guidance. Small pleural effusion size (measuring <1 cm on a lateral decubitus radiograph, which corresponds to an approximate volume <300 mL) is a relative contraindication to thoracentesis. However, the percutaneous sampling of small effusions can provide essential diagnostic information. If a small effusion is identified, performing thoracentesis with image guidance can increase the success of sampling and minimize complications.
Pleural effusion volume can be estimated by plain radiography and CT. The minimum fluid volume that can be detected on a lateral decubitus radiograph is approximately 10 mL, and therefore any visible pleural effusion must measure at least 10 mL in volume. On the lateral projection, blunting of the posterior costophrenic angle occurs with an effusion volume of at least 50 mL. On the posteroanterior (PA) view, at least 200 mL of fluid is needed to obscure the lateral costophrenic angle, and at least 500 mL is required to obscure the normal diaphragmatic contour. On CT, effusion volume can be estimated on the axial image superior to the hemidiaphragm in which the effusion appears largest. Effusions occupying less than 25% of the anteroposterior (AP) dimension of the hemithorax, or measuring less than 3 cm in AP dimension in the midclavicular line, correspond to a volume less than 300 mL. Effusions occupying more than 50% of the hemithorax, or more than 10 cm in AP dimension, have a volume more than 1000 mL.
Characterization of Effusions on CT
CT is well suited for the spatial characterization of pleural effusion and can accurately define the location and extent of pleural effusions. Free-flowing pleural fluid layers dependently and often has a crescentic shape in the axial plane. Loculated effusions, in contrast, have a lenticular shape and can displace adjacent lung parenchyma. In challenging cases, additional imaging in the prone or decubitus position can help differentiate simple effusions from loculated effusions.
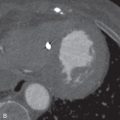
Although some specific types of pleural effusion can be readily diagnosed by CT, in general, the modality is limited in characterizing the cause of pleural fluid. Acute hemothorax is most easily identified by CT and demonstrates high attenuation, usually from 35 to 70 Hounsfield units (HU) ( Fig. 35.3 ). Occasionally, a hematocrit effect (a fluid-fluid layer with higher density in the dependent layer) is seen. As hemothorax evolves, fluid attenuation more closely approximates water and is more difficult to discriminate from other causes of effusion. Chylothorax rarely can present as a low-attenuation effusion (<0 HU) or with a fat-fluid layer. However, its attenuation usually approximates water due to an averaging of fat and protein. CT cannot reliably differentiate exudative and transudative effusions. Although certain features, such as loculation, pleural thickening, and pleural nodularity, are more common in exudative effusions, the findings are nonspecific and cannot replace pleural fluid laboratory analysis.

▪
Empyema
Imaging Features of Empyema
Empyema usually presents as a pleural effusion in a febrile patient. The most common imaging appearance of empyema is an uncomplicated pleural effusion. Loculated pleural effusions are less common but are an important sign of increased complexity that should elevate the level of suspicion for infection, particularly in a patient with persistent fever and leukocytosis of unknown origin. On CT, the visceral and parietal pleura surrounding each side of the empyema are visible and uniformly thickened, a finding known as the split pleura sign . Both pleural layers typically enhance with the administration of IV contrast.
Effect of Multiloculation on Management
Treatment of empyema includes an extended course of antibiotics and percutaneous drainage of pus. It is important to identify and describe every loculated collection seen because each one will likely require separate drainage. If the patient is reimaged after draining thoracostomy tubes are inserted, careful attention should be paid to any undrained or inadequately drained collections. Occasionally, a single locule can require multiple tubes due to high-viscosity pus or septations that cannot be visualized by CT.
Significance of Air in an Empyema
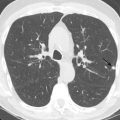
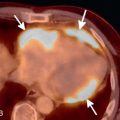
Spontaneous air in an empyema (or any pleural effusion) indicates the presence of a bronchopleural fistula ( Fig. 35.4 ). A bronchopleural fistula may occur as a complication of infection (particularly tuberculosis), recurrent spontaneous pneumothorax, or chemoradiation for lung cancer. If a bronchopleural fistula is present, adequate drainage of the empyema is essential to ensure adequate healing and prevent back-spillage into the bronchial tree, which can result in diffuse pneumonitis. Occasionally, tube thoracostomy is insufficient, and surgical drainage or repair is required. Bronchopleural fistula may also occur as a postoperative complication following pneumonectomy when an air leak at the bronchial stump communicates with the pleural space.

▪
Pneumothorax
Signs of Tension Pneumothorax
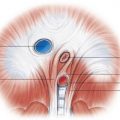
Tension pneumothorax refers to an increasing accumulation of air within the pleural space (usually increasing with inspiration, without egress of air from the pleural space on expiration) causing contralateral mediastinal displacement and, ultimately, leading to respiratory failure from compression of the ipsilateral and contralateral lungs and diminished return of blood to the right heart. It is primarily a clinical diagnosis, but there are several radiographic findings associated with tension pneumothorax that should prompt urgent communication with the referring provider. Any mass effect on the mediastinum, such as a contralateral mediastinal shift or tracheal deviation, should raise suspicion for tension pneumothorax ( Fig. 35.5 ). However, in patients maintained on positive-pressure ventilation, mediastinal shift may not be evident, even if tension pneumothorax is present. Inversion of the ipsilateral hemidiaphragm or a widened ipsilateral intercostal space are also concerning signs of increasing intrathoracic pressure and tension. The degree of ipsilateral lung collapse is not a reliable sign of tension because underlying lung disease or pleural scarring can prevent collapse of some areas of the lung.

Importance of Apical Emphysema or Apical Bullae
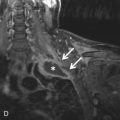
Prominent apical emphysema or apical bullae predispose a patient to spontaneous pneumothorax, which can be recurrent. These patients often benefit from bullectomy (in combination with pleurodesis), so identification of these dilated air spaces can help guide management and prevent future pneumothoraces. Also, the identification of concurrent contralateral bullae will affect surgical planning because both sides may be targeted for surgical treatment. Apical bullae are usually well visualized in the coronal plane on CT. Placement of a thoracostomy tube into an apical bulla can cause a bronchopleural fistula and should be avoided.
▪
Lipoma and Liposarcoma
Imaging Features Concerning for Liposarcoma
Lipomas are the most common benign tumor of the chest wall and diaphragm and typically present as incidental findings in asymptomatic patients. Most lipomas are composed almost entirely of fat, have a thin capsule, and may have thin internal fibrous bands. Any thickened bands of soft tissue attenuation, nodular soft tissue, or large soft tissue components within a fatty mass are suspicious for liposarcoma, a relatively rare malignant tumor of fat. These findings are best assessed by CT, and the soft tissue components often enhance with IV contrast. Large fat-containing lesions must also raise suspicion of malignancy, although lipomas can also grow to a large size over time.
Usefulness of MRI
MRI can characterize soft tissue accurately and will demonstrate similar findings to those seen on CT. Fat components are easily differentiated from soft tissue components. Fat demonstrates T1 and T2 hyperintensity, with loss of signal on fat saturation sequences. Soft tissue has variable T1 and T2 signals but will enhance with gadolinium contrast. MRI can also be used to define the extent of involvement for surgical planning. If CT findings are indeterminate, MRI is useful for identifying other masses, such as intramuscular neurofibromas, and for evaluating the extent of neurogenic tumors.
▪
Asbestos-Related Pleural Disease
Types of Benign Asbestos-Related Pleural Disease
Although prior asbestos exposure is concerning due to significantly increased risks of interstitial lung disease, lung cancer, and malignant mesothelioma, benign asbestos-related pleural disease is far more common. The four types of benign disease are noncalcified pleural plaques, calcified pleural plaques, benign asbestos pleural effusion, and diffuse pleural fibrosis.
Pleural plaques are the most common of the benign entities and develop approximately 20 to 30 years after the initial asbestos exposure. The plaques are composed of fibrohyaline tissue and form within the parietal pleura, manifesting with characteristic radiographic and CT findings ( Fig. 35.6 ). On radiography, pleural plaques manifest as discontinuous areas of pleural thickening that may be calcified or noncalcified, with incomplete borders, and characteristically distributed along the costal, diaphragmatic, and paravertebral pleural surfaces. They typically spare the pleural surfaces in the lung apices and costophrenic sulci.