3 The renal tract
EMBRYOLOGY
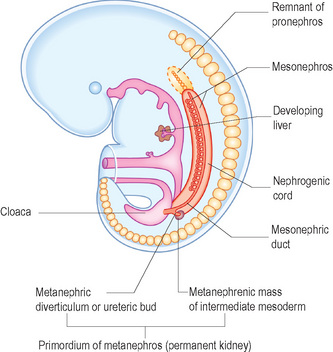
Three sets of excretory organs develop in a human embryo in a cranial to caudal progression (Fig. 3.1):
Metanephroi
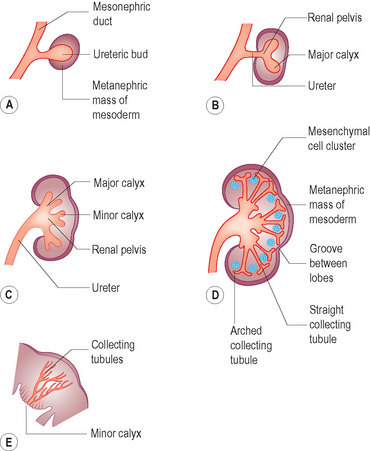
• The ureteric bud is an outgrowth from the mesonephric duct close to its entry into the fetal cloaca. The ureteric bud goes on to develop the ureter, renal pelvis, calyces and collecting tubules. The collecting tubules undergo repeated branching, which induces clusters of mesenchymal cells in the metanephric mass of mesoderm. These go on to form the metanephric tubules, which ultimately go on to form the nephron (Fig. 3.2).
• The metanephric mass of intermediate mesoderm. The nephron is derived from the metanephric mass.
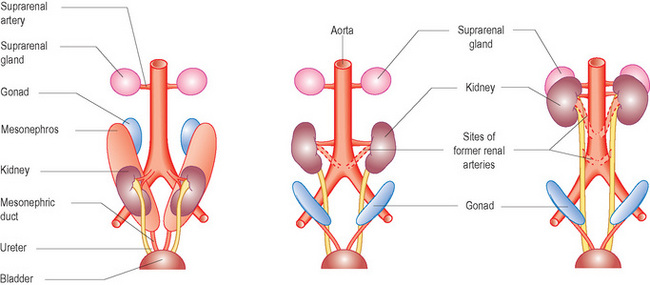
Initially the permanent kidneys lie close to each other in the pelvis. As the abdomen grows, the kidneys move cranially (towards the head) and gradually move further apart. Eventually they come to lie in the retroperitoneum on the posterior abdominal wall. As they ascend from the pelvis they receive a higher and higher blood supply so that eventually they receive blood supplied from the aorta. This also accounts for the multiple renal arteries which often supply the kidneys. When they come into contact with the adrenal gland the ascent stops (Fig. 3.3).
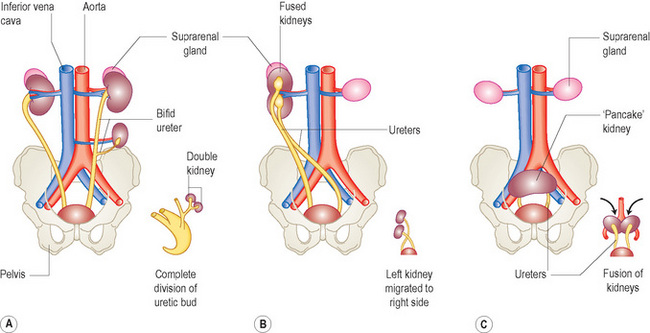
Figure 3.4 gives a schematic diagram of some of the more common anomalies of the urinary system. Renal anomalies occur in some 3–4% of newborn infants and are usually those of number of kidneys, rotation and position.
RENAL ANOMALIES
Anomalies of the upper urinary tract
Renal agenesis
Unilateral renal agenesis is relatively common, occurring in about 1 in 1000 newborn infants. Some cases may, in fact, be as a result of an involuted multicystic kidney in later life. Males are more affected than females and the left kidney is most commonly the one that is absent. The contralateral kidney usually undergoes compensatory hypertrophy. Unilateral renal agenesis should be suspected in infants with a single umbilical artery (Fig. 3.5).
Abnormalities of position
Ectopic kidneys
One or both kidneys may be in an abnormal position if they fail to progress along their normal migratory path. Most kidneys are located in the pelvis, in which case they may fuse to form a pancake kidney. Ectopic kidneys may present as a mass and some have been inadvertently removed. Occasionally a kidney may continue to migrate cranially, in which case it may be found in the chest. Ectopic kidneys receive their blood supply from blood vessels near them (for example, iliac arteries if they lie in the pelvis) and are often supplied by multiple vessels (Fig. 3.6).
Crossed-fused ectopia
When one kidney is displaced across the midline and fused inferiorly to another normally positioned kidney this is termed cross-fused ectopia. The lower kidney’s (i.e. displaced kidney) ureter enters the bladder normally on the contralateral side. These kidneys are prone to develop obstructive uropathy and there is also an increased incidence of vesicoureteric reflux (VUR). Crossed-fused ectopia and single kidneys are the renal anomalies most commonly associated with the VACTERL association (Fig. 3.7).
Anomalies of the lower urinary tract
Anomalies of the distal ureter
Primary megaureter
Megaureter is a general term used to describe the presence of a dilated ureter with or without associated dilation of the upper collecting system. The normal ureter in children is rarely more than 5 mm1 and if a ureter is detected on ultrasound is generally considered to be abnormal. The term primary megaureter is a term that includes all abnormalities related to a congenital alteration at the vesicoureteric junction (VUJ) and may be obstructed, refluxing or non-refluxing non-obstructed.
A refluxing megaureter is due to a short or absent intravesical ureter or an abnormality of the VUJ.
Ureterocele
On ultrasound the ureterocele appears as a cystic structure with a thin membrane within the bladder almost always associated with a dilated ureter. If large enough it will obstruct the bladder, and in boys may even be mistaken for PUV with an irregular trabeculated bladder wall. In nearly three quarters of pediatric patients an ectopic ureterocele is associated with a duplex collecting system and is the distal portion of the upper moiety ureter. The upper moiety is usually obstructed by the ureterocele. Due to the stasis of urine, urinary tract infections and stones in the ureterocele may occur. VUR may be associated with the lower moiety duplex due to the shorter and more vertical intravesical course of the ureter as mentioned previously (Fig. 3.8)
Primary vesicoureteric reflux (VUR)
Vesicoureteric reflux is the flow of urine from the bladder into the ureter and upper collecting system. It may be isolated but is commonly associated with other abnormalities, in particular bladder and bladder outflow obstructions. There is also a known association with duplex systems and multicystic kidneys, and it is known to be a familial condition (Table 3.1).
Table 3.1 Grades of VUR based on guidelines of the International Reflux Study Committee20(Lebowitz)
| Grade | Characteristics |
|---|---|
| I | Ureter only |
| II | Ureter, pelvis, and calyces; no dilation; normal calyceal fornices |
| III | Mild or moderate dilation or tortuosity of the ureter and moderate dilation of the renal pelvis; no or slight blunting of the fornices |
| IV | Moderate dilation or tortuosity of the ureter and moderate dilation of the renal pelvis and calyces; complete obliteration of the sharp angle of the fornices but maintenance of the papillary impressions in the majority of calyces |
| V | Gross dilation and tortuosity of the ureter; gross dilation of the renal pelvis and calyces; papillary impressions are no longer visible in the majority of the calyces |
Anomalies of the bladder
Bladder agenesis is extremely rare and most infants are stillborn.
Urethral abnormalities
Posterior urethral valves
This is the commonest urethral abnormality occurring in boys. There is a thick membrane in the posterior urethra which behaves as a valve so that while a catheter can be passed into the bladder, the infant is unable to adequately void and empty the bladder. This causes a typical dilation of the posterior urethra; i.e. dilation proximal to the obstructing valve (Fig. 3.9).
ULTRASOUND PREPARATION AND TECHNIQUE
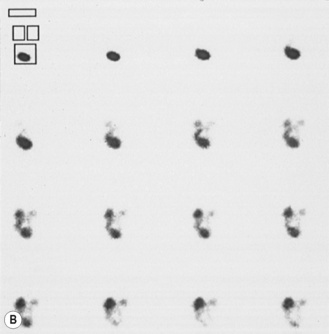
The anatomical information given by ultrasound is independent of function. Functional information is generally obtained from nuclear medicine studies. Sometimes further anatomical information, particularly of the ureters and calyces, is needed and for this an intravenous urogram (IVU) is performed (Table 3.2) (Fig. 3.10).
Table 3.2 Renal investigations in children
| Investigation | Description | Indication |
|---|---|---|
| Micturating cystourethrogram (MCU) | Bladder catheterization. Demonstrates bladder size and shape. VUR if present. Only test which will show urethral abnormalities in boys | |
| Direct isotope cystogram (DIC) | Bladder catheterization and isotope contrast | First investigation in girls and for follow-up in toddlers who are not potty- trained. Lower radiation dose than IRC |
| Voiding urosonography | Bladder catheterization and ultrasound contrast | |
| Indirect radioisotope cystograms (IRC) | Dynamic renogram followed by voiding to look for VUR | Follow-up of VUR in older continent children. Gives functional information about kidneys |
| Dimercaptosuccinic acid (Tc 99m DMSA) | Isotope gets fixed in the kidney | |
| Diethylenetriaminepentaacetate (Tc 99m DTPA) | Dynamic renogram | Assess differential renal function and drainage |
| Mercaptoacetyltriglycine (Tc 99m MAG3) | Isotope taken up by kidney and passed in urine. After 30 min micturition views to look for VUR (IRC) | Postoperative evaluation of the collecting system: Indirect cystography Following renal transplantation Renography with captopril stimulation for renovascular hypertension |
| Intravenous urogram (IVU) | Contrast injected intravenously. Series of views of the kidneys and bladder as the contrast passes through the system |
IRC, indirect radioisotope cystogram; PUV, posterior urethral valves; UTI, urinary tract infection; VUR, vesicoureteric reflux.
Preparation
Start with the patient supine and examine the bladder first as the infant may micturate and vital information will be lost (Box 3.1).
BOX 3.2 Normal renal tract ultrasound series
• Supine longitudinal and transverse full bladder views. Bladder wall measurements away from the trigone
• Post-micturition supine longitudinal of the right kidney and left kidney comparing the echogenicity to the liver and spleen
• Prone bipolar measurement of maximal length of both kidneys
• Prone transverse pelvic diameter at the point of maximal width (either intra- or extrarenal). Use Doppler to identify the renal vessels
• Supine post-micturition longitudinal and transverse views of the bladder and a volume measurement of post-micturition residue if present
An overfull bladder may cause a mild fullness of the collecting system, so it is best to start the examination of the full bladder and then get the patient to micturate. After micturition, examine the kidneys. If the kidneys appear dilated with the full bladder, then it is generally best to wait at least 15 minutes before examining the kidneys again. Establish a local protocol so that the referring nephrourologists know exactly when (i.e. before or after micturition) and where the renal pelvis is measured (Box 3.2)
BOX 3.1 Tips
• Always start with the bladder first
• If the kidneys are tucked underneath the ribs try using deep inspiration or expiration, or by asking the child to blow their tummy up like a balloon, i.e. Valsalva
• Dilated ureters in a neonate are best seen in the supine position
• Try scanning the upper ureter by angling the probe 45° medial of the maximum renal length in the prone position
• Bipolar length must be measured prone. Supine renal lengths may be falsely high due to image magnification
• Bladder wall should be measured away from the trigone as the musculature here is slightly thicker than the rest of the bladder wall and may give falsely high measurements
• A renal ultrasound examination is incomplete if the bladder has not been examined full and after micturition
Doppler evaluation is integral to the examination but particularly important in conditions such as:
• renal transplant assessment (to evaluate blood flow to the kidney and areas of hypo- and hyperperfusion)
• essential in primary non-function of the renal transplant
• suspected renal vein thrombosis (to evaluate the renal vein and inferior rena cava)
• renal tumor (to evaluate renal vein and IVC for tumor thrombus)
• hypertension (to look at the caliber of the aorta, aneurysmal dilation of the main renal vessels and particularly to assess the intrarenal spectral trace).
Normal ultrasound appearances
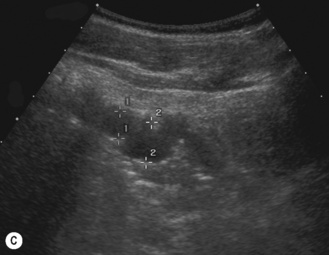
After 6 months and in the older child the echogenicity of the cortex becomes more hypoechoic and the cortex appears thicker in relation to the medullae. The central sinus echoes become more prominent with age and body fat. Normally no calyceal dilation is seen. The renal pelvis shows some variation in size and a transverse pelvic diameter of <10 mm is considered to be within the normal range. Good hydration of the child and a very full bladder may result in it being more prominent1 (Figs 3.11 and 3.12).
When imaging the renal tract it is important to have the children fully hydrated in order to accurately assess any degree of pelvicalyceal dilation. A grading system of hydronephrosis has been proposed2 such that:
• Grade 1—renal pelvis only visualized
• Grade 2—hydronephrosis is present when a few but all not all calyces are identified in addition to the renal pelvis
• Grade 3—hydronephrosis requires that all calyces are seen
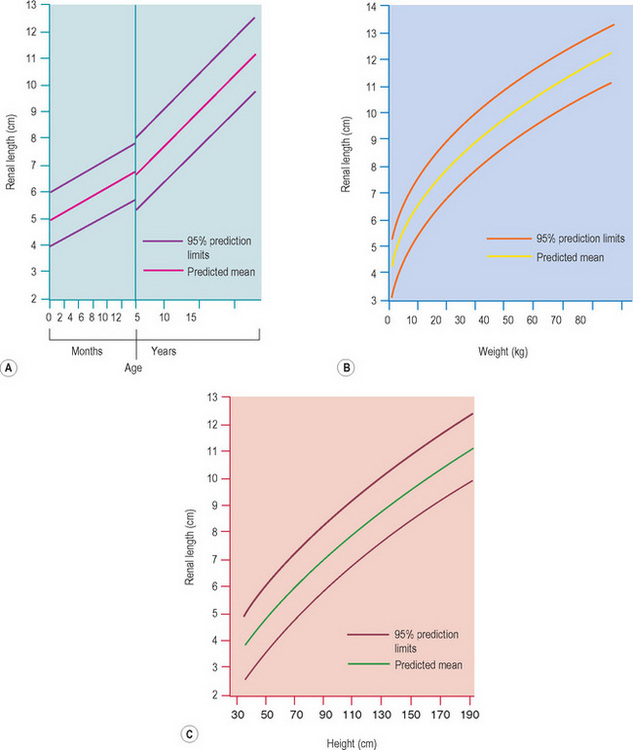
Normative charts must be available and used for all examinations. The normal renal length relative to age should be quoted in all reports. However, renal patients are often poorly grown and small so that it is our preference to use charts that also allow us to compare weight and height rather than age alone. The length of the kidney is the easiest parameter to assess but volume has a more accurate correlation with most body size parameters (i.e. weight and height, which is important in children) The renal length in normal term neonates is 3.4–5.0 cm and the renal volume is 5.7–14.3 cm3. As a rule of thumb in premature infants use 1 mm renal length per week gestation in order to assess renal size.3–5 In children who have had a kidney removed or only have a single functioning kidney, due to a multicystic dysplastic kidney for example, use charts for a single kidney (Fig. 3.13).6
The normal bladder wall when distended is 0.04–0.27 cm (90–100% of capacity) and when empty is 0.16–0.39 cm (0–10% of capacity) (Fig. 3.14).7
In little girls a normal phenomenon occurs of vaginal filling post-micturition. This is occasionally observed in cystography and is a normal finding (Fig. 3.15).
Doppler examination
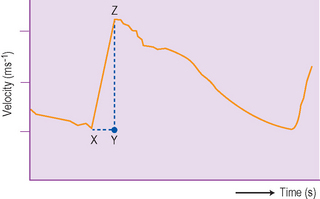
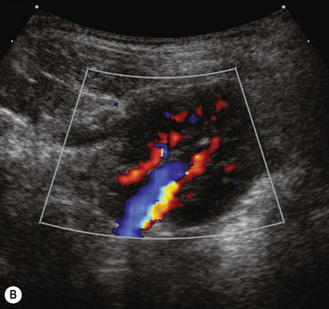
The color Doppler should be used as an adjunct when assessing the renal pelvis and hilar vessels and for a quick overview of the blood flow to a kidney. It is particularly useful in renal transplantation. It should be used in suspected renovascular hypertension both to evaluate the aorta and the kidneys. The appearance of the normal intrarenal Doppler waveform is shown and reflects the low resistance of the renal bed (Fig. 3.16).
PRENATALLY DIAGNOSED UROLOGICAL ABNORMALITIES
Since the introduction of routine prenatal scanning and latterly more detailed anomaly scanning, the prenatal ultrasound diagnosis of renal abnormalities is now well established. This prenatal detection has resulted in a whole new group of patients presenting to pediatric urologists in the last decade. These infants are primarily asymptomatic, and are referred for urological opinion and radiological investigation and monitoring (see Ch. 2). Treatment is mainly preventive and relies on close follow-up and timely intervention when necessary.
The role of ultrasound postnatally is to:
The differential diagnosis is listed below and the echogenic or bright kidney will be dealt with later (Table 3.3) (Box 3.3).
Table 3.3 Differential diagnosis of prenatal hydronephrosis
| Unilateral | Bilateral |
|---|---|
| RPD | RPD |
| VUR | VUR |
| Megaureter (± VUR) | Megaureters (± VUR) |
| MCK (simple) | MCK (complicated); cystic dysplastic or dilated (PUJ) kidney on the opposite side |
| Complicated duplex kidney, i.e. obstructed upper moiety with ureterocele and/or refluxing lower moiety | Complicated duplex kidneys |
| Bladder or outlet pathology, e.g. neurogenic bladder or PUV with bilateral upper tract dilation |
RPD, renal pelvic dilation; VUR, vesicoureteric reflux; MCK, multicystic dysplastic kidney; PUJ, pelviureteric junction; PUV, posterior urethral valves.
BOX 3.3 General concepts for postnatal imaging protocol
• Use of antibiotics in all patients is controversial. Some advocate prophylactic trimethoprim at least until VUR has been excluded
• Postnatal ultrasound after 2–3 days when the infant is well hydrated
• Follow-up examination after 6 weeks to confirm the perinatal ultrasound findings
• Urgent urological referral for infants suspected of having PUV and complex duplex systems
• Conventional contrast cystography on dilated ureters, duplex systems and suspected PUV ‘thick-walled bladders’
• Functional imaging generally after 3 months (after period of transitional nephrology), usually MAG3
Prenatal detection of renal pelvic dilation and normal postnatal scan
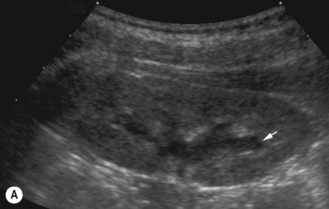
The commonest abnormality in general obstetric practise is mild renal pelvic dilation which on postnatal ultrasound is found to be normal. This is the largest group of children detected: 30% have been shown to have vesicoureteric reflux and it is for this reason that some centers advocate the routine use of cystography. However, this is controversial as it imposes a large workload on radiology departments and some argue that there will be too many false-negative results as VUR resolves anyway. Long-term studies do not exist in this group but it would appear that while vesicoureteric reflux exists, these kidneys are normal and not scarred or dysplastic. However, their potential for renal damage in the presence of infection must be high (Fig. 3.17).
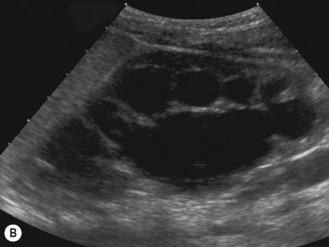
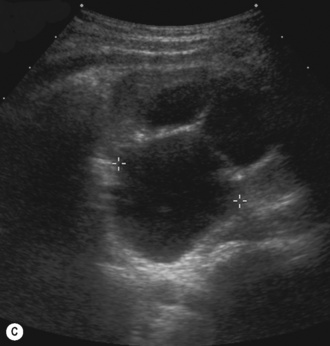
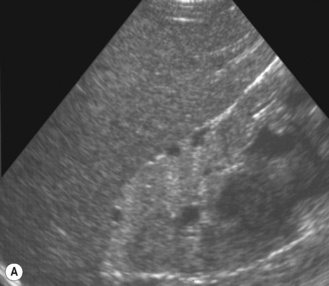
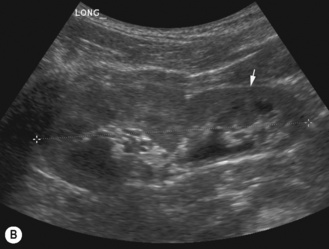
There is another group of patients who have an prenatal diagnosis of hydronephrosis but whose kidneys are not entirely normal on ultrasound, with irregular outlines and areas of cortical thinning, increased echogenicity and dilation of the collecting system. These patients appear to have damaged or dysplastic kidneys in the presence of prenatal vesicoureteric reflux and have a different prognosis with a much higher potential for further renal damage should infection occur (Fig. 3.18).8–13
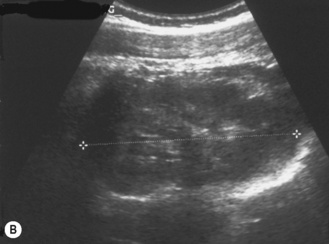
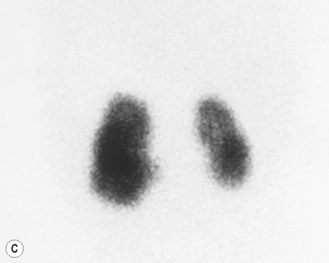
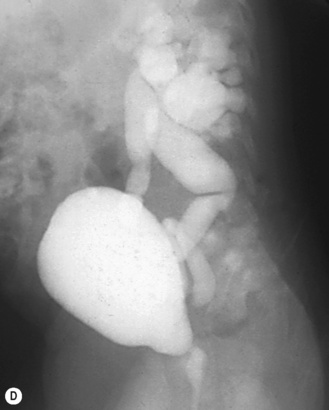
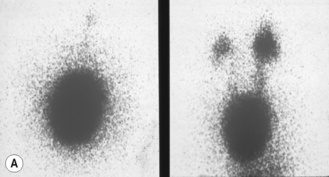
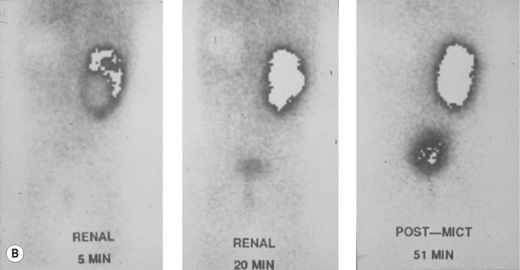
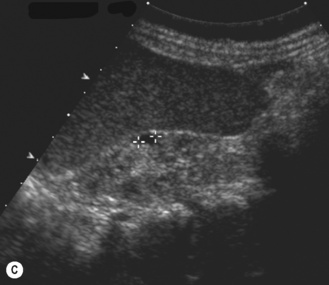
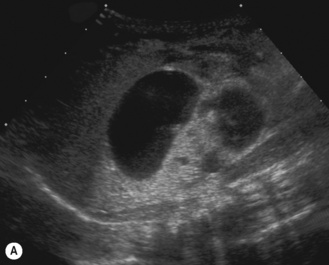
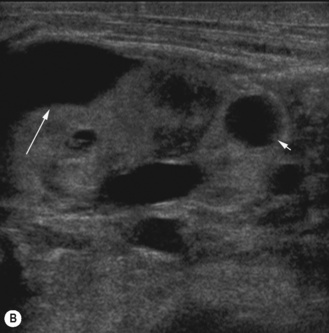
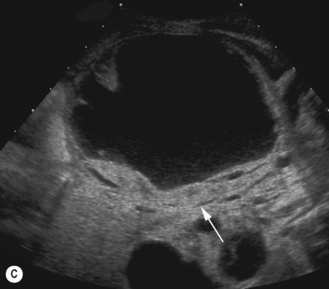
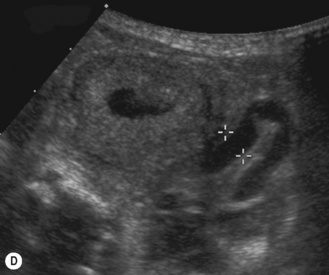
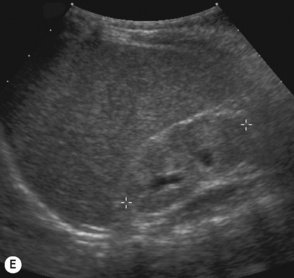
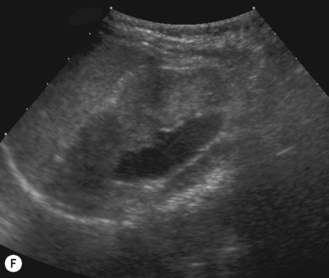
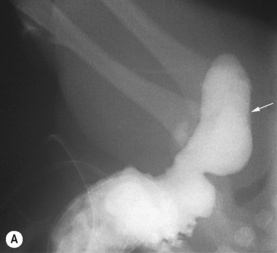
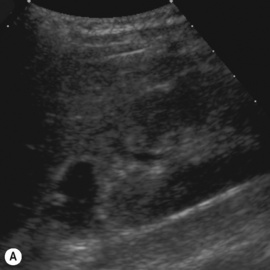
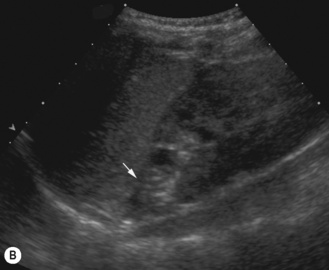
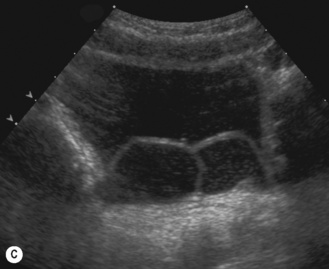
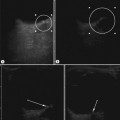
Figure 3.18 Abnormal kidney and vesicoureteric reflux (VUR). (A) & (B) Longitudinal sonogram of left and right kidneys in an infant with prenatally diagnosed renal tract dilation. There is asymmetry in size of the kidneys and abnormal outline. (C) DMSA (see Table 3.2) on the same infant showing the grossly abnormal renal outlines of both kidneys. The right kidney is smaller than the left. (D) There was bilateral VUR at cystography. These prenatally diagnosed infants are a well-defined group and have never had a urinary infection. This is termed fetal reflux nephropathy and they are at greater risk of long-term renal damage if infection occurs.
Interestingly, this whole group is more common in male infants.14 Further imaging will depend on management and the postnatal ultrasound examination. Depending on local protocols, in some centers contrast cystography will be performed on these infants. Those with VUR will be placed on antibiotic cover and have a DMSA performed. If after the normal postnatal ultrasound no further imaging including a cystogram is undertaken, then it is reasonable for these children to be placed on antibiotic prophylaxis at least for the first year of life while the potential for renal damage is at its highest.
Unilateral pelvic dilation
Mild renal pelvic dilation is one of the commonest prenatal diagnoses the sonographer will encounter. When reporting the examination it should be clearly stated whether there is calyceal dilation and the size of the renal pelvis. Simply calling the dilation a hydronephrosis is not sufficient and poorly describes the state of the collecting system. This is particularly important for monitoring and accurate long-term follow-up. Care should be taken in not mistaking the renal pelvis for renal vasculature at the hilum and a quick Doppler to confirm the position of the vasculature is sometimes needed (Fig. 3.19).15,16
A renal pelvic diameter of 10 mm and less is generally considered to be within the normal range.17 Isolated RPD <10 mm is virtually always benign and no aggressive investigations should be undertaken. Urologists vary but some would even take renal pelvic diameters of <15 mm to be benign and treat conservatively. Most would not undertake further imaging and would simply follow with ultrasound examinations. However, if there is calyceal dilation the prognosis for the kidney is generally worse and the threshold for imaging is lower. It is essential that the sonographer is able to recognize calyceal dilation. Calyceal and/or ureteric dilation irrespective of renal pelvis diameter are always significant and both reflux and obstruction must be excluded. This dilation of the renal pelvis is sometimes called PUJ (pelviureteric junction) obstruction (Fig. 3.20).18
Bilateral renal pelvic dilation
Mild renal pelvic dilation may be bilateral. No general agreement on how to manage these patients exists. Postnatal imaging should be the same as that for unilateral renal pelvic dilation. Some centers include a cystogram (Table 3.4).
Table 3.4 Imaging protocol for RPD
| Unilateral | Bilateral |
|---|---|
| Postnatal ultrasound after 2–3 days | Same as unilateral but add MCUG |
| MAG3 renogram after 2–3 months if RPD > 15 mm | |
| No MCUG if no dilated ureter |
MCU, micturating cystourethrogram.
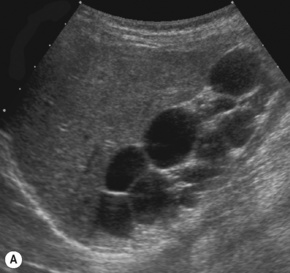
Multicystic kidney (MCK)
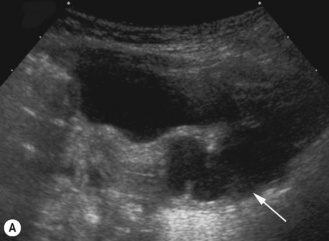
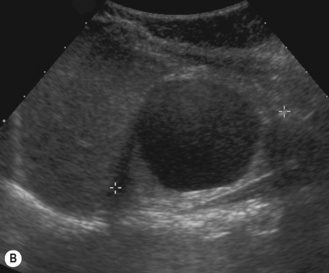
This is a non-hereditary cystic kidney which is sometimes otherwise known as multicystic dysplastic kidney. The characteristic feature of a multicystic kidney is the atretic ureter, and there is no continuity between the glomerulus and the calyces. On functional radionuclide studies they are non-functioning. The condition is more common in males and the natural history is to involute with time either prenatally or postnatally. Urological opinion varies and nowadays these kidneys are not removed unless they are causing symptoms or evidence of respiratory embarrassment. There have been reported associations with hypertension and malignancy, although generally these are not used as indications for early removal of the MCK (Fig. 3.21).
Ultrasound and postnatal imaging
The contralateral kidney should be carefully examined, looking for dilation of the calyces, pelvis or ureter and increased parenchymal echogenicity which may reflect renal dysplasia (Fig. 3.22).
In some centers a DMSA or MAG3 study is performed in order to document the normality of the contralateral kidney. This is also controversial and some would only perform these examinations on the abnormal (so-called ‘complicated’) contralateral kidney detected by ultrasound (Table 3.5).
Table 3.5 Imaging protocol for MCK
| Simple | Complicated |
|---|---|
Posterior urethral valves
The presence of bilateral hydronephrosis and a full bladder during intrauterine life associated with oligohydramnios is the essential prenatal diagnosis (see Ch. 2). These infants need urgent postnatal imaging in order to make the diagnosis and allow early treatment of the bladder outflow obstruction. Also these infants have a high risk of developing a urinary tract infection which could further compromise the kidneys and even prove fatal.
Postnatal ultrasound findings would include bilateral hydronephrosis and hydroureters, echogenic cystic dysplastic kidneys, a urinoma where the urine has ruptured out of the kidney from a calyx and a thick-walled bladder (Fig. 3.23).
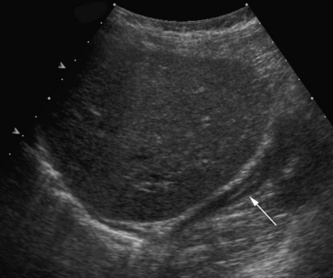
Thick-walled bladder
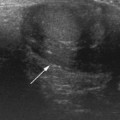
In suspected PUV a longitudinal ultrasound examination of the perineum using a high-frequency linear transducer will be able to demonstrate the thick-walled bladder base and a dilated posterior urethra. This should be done when the infant is voiding so that there is maximal dilation of the posterior urethra. Dilation of the posterior urethra measuring more than 7 mm is abnormal (Fig. 3.24).
The infant suspected of having posterior urethral valves should have an urgent MCUG.
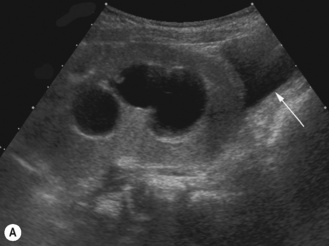
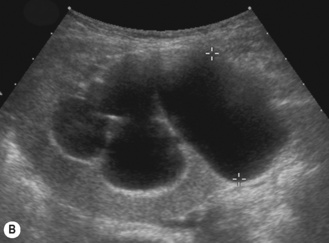
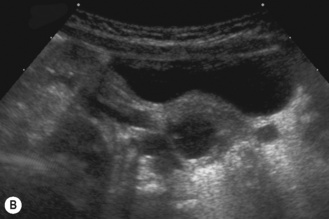
The duplex kidney
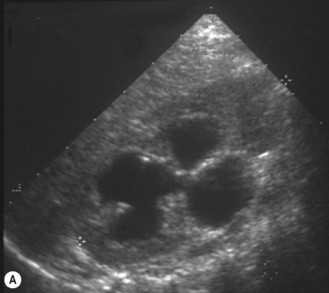
The duplex kidney can be very easy to diagnose but is also one of the most difficult and complex conditions, requiring the use of all imaging modalities. However, ultrasound plays an important diagnostic role and the sonographer needs to be aware of all the variations in its appearance and clinical presentation. The dilated systems with ureteroceles tend to be detected prenatally but older children may present with urinary tract infections and girls with constant 24-hour wetting (Fig. 3.25).
Ultrasonically there are a number of features to look for:
• The duplex kidney may be longer than the contralateral kidney. (A bifid renal pelvis is considered to be a normal variation—Fig. 3.26.)
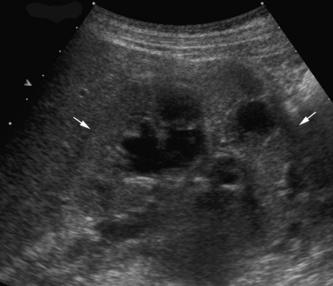
• The upper moiety obstructs and is associated with either a ureterocele in the bladder or ectopic drainage, for instance into the vagina. The ureters must be carefully examined: in particular in a girl, for entry into the vagina. The vagina full of fluid in a suspected duplex situation is an important finding.
• The upper moiety may have small peripheral cortical cysts and evidence of increased echogenicity and dysplasia.
• In a small cryptic duplex system the only finding may be a dilated ureter from the tiny upper moiety, which is often best detected on the prone views of the kidney as a tubular structure passing anterior to the kidney.
• The lower moiety dilates, which is usually vesicoureteric reflux but may be renal pelvic dilation alone.
On functional imaging there may be equal function between upper and lower moiety. In some, the upper moiety function may be reduced due to dysplasia or obstruction. Lower moiety function may also be reduced due to scarring from the vesicoureteric reflux (Fig. 3.27). Cystograms must be performed. Nuclear medicine studies such as DMSA and MAG3 are performed to show a differential function and drainage. IVU is generally not needed in all duplex systems. However when the ultrasound and nuclear medicine studies do not entirely complement each other, this may then necessitate a carefully performed IVU.