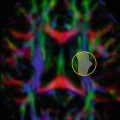
Fig. 16.1
An example of diffusion tensor imaging (DTI) tractography analysis (diffusion toolkit http://trackvis.org/dtk) of a healthy control (HC) (left side) and a patient with secondary progressive multiple sclerosis (MS) (right side). The B0 image and fractional anisotropy (FA) map are shown in the top part of the figure. In the middle part, the global reconstruction of all fibers is shown. After the definition of a region of interest (ROI) for tracking the corpus callosum (CC) on the FA map, the reconstruction of the CC is shown at the bottom. In the MS patient, several periventricular lesions, causing a reduction of FA values, are visible on the B0 image. Diffuse atrophy is also evident (enlargement of the ventricles and thinning of the CC on the midsagittal slice). The reconstruction of the CC shows the interruption of fiber propagation due to the presence of low FA values in lesions. Parameters used for tractography: FA threshold = 0.15, angle threshold = 35°, propagation algorithm: FACT with spline filter applied
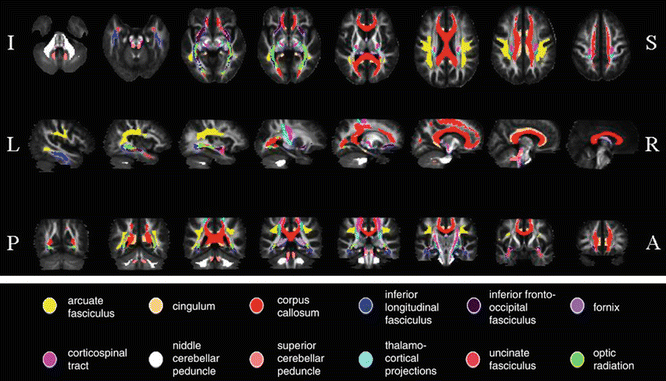
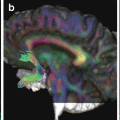
Fig. 16.2
White matter (WM) tract probability maps obtained from a reference group of 24 healthy controls. Probability maps are superimposed on axial (top row), sagittal (middle row), and coronal (bottom row) sections of the fractional anisotropy (FA) atlas. A anterior, I inferior, L left, P posterior, R right, S superior. Images are presented in neurologic convention. [Adapted from Preziosa P, Rocca MA, et al. (2011). “Intrinsic damage to the major white matter tracts in patients with different clinical phenotypes of multiple sclerosis: a voxelwise diffusion-tensor MR study.” Radiology 2011;260(2):541–50. With permission from The Radiological Society of North America]
Likewise, voxel-based analysis depends strongly on nonlinear registration methods and on their capability to produce an adequate overlap between subjects. However, the application of a nonlinear deformation introduces new issues related on how to use additional pieces of information on tract morphology in order to drive the registration. This is the consequence of an increased ability of this algorithm to compensate for local morphological differences. It has been shown that the accuracy of registration is higher when the whole DTI acquisition set is used to drive the transformation. For instance, the use of six channels of tensor components allows matching of the shape, magnitude, and orientation of local tensors between images better than that of one channel of FA or one channel of T2-weighted image.
Interpretation of DTI Findings
The interpretation of DTI findings is not straightforward. So far, the actual pathological features underlying diffusion abnormalities in MS are not completely understood. Some pieces of evidence suggest that the various, and often concomitant, pathological abnormalities occurring in MS (i.e., inflammation, demyelination, axonal loss, Wallerian degeneration) can affect diffusivity and anisotropic characteristics of tissues in opposing ways, thereby complicating the interpretation of DTI findings [1, 2, 11].
DTI Features of MS
Focal WM Lesions
DTI can contribute to grade the severity of damage within focal MS lesions, which typically show increased mean diffusivity (MD) and decreased fractional anisotropy (FA). However, abnormalities of DTI indexes are highly heterogeneous among different T2 lesions [3, 4]. The more severe abnormalities are found in T1 hypointense lesions (the so-called black holes) [12–14], which represent areas of irreversible tissue disruption, gliosis, and axonal loss. Conversely, conflicting results have been reported when comparing findings in gadolinium-enhancing vs. nonenhancing lesions [12–15], although most of the studies found lower FA values in enhancing lesions [14, 16]. These findings suggest that DTI might grade intrinsic abnormalities of acute lesions, which in turn are likely to reflect different substrates, some of which are transient (e.g., edema, demyelination, and remyelination) and others, permanent (e.g., neurodegeneration and axonal loss). Moreover, a longitudinal study of enhancing MS lesions followed up for 1–3 months [15] showed that MD values were increased in all lesions, but continued to increase during follow-up only in a subgroup of them. This finding highlights the notion that contrast enhancement does not allow the differentiation of acute MS lesions, which might be characterized by varying degrees of tissue disruption.
NAWM
Using different methods of analysis, increased MD and decreased FA have been consistently found in the NAWM of MS patients [3, 4], even before the formation of new focal lesions [20]. Such abnormalities can be detected from the earliest stages of the disease, also in patients with clinically isolated syndrome (CIS) suggestive of MS [21, 22], and become more pronounced with increasing disease duration and neurological impairment [3, 4]. NAWM damage in MS patients is distributed but tends to be more severe in perilesional areas [23, 24] and at sites where MS lesions are typically located [14, 25].
NAWM damage is only partially correlated with the extent of T2 lesions and the severity of intrinsic lesion damage [3], which suggests that diffusivity changes in NAWM are not entirely dependent on retrograde degeneration of axons transected in focal lesions, but they rather represent diffuse abnormalities beyond the resolution of conventional MRI. A study [26] correlated diffusivity with perfusion findings in the corpus callosum of patients with relapsing-remitting (RR) MS . These results are more consistent with a primary ischemia than a secondary hypoperfusion due to Wallerian degeneration. These findings suggest a potentially reversible substrate of tissue damage, related to vascular impairment, whereas hypometabolism from axonal degeneration would represent an advanced and irreversible condition.
DTI is also useful for assessing the evolution of MS damage over time [21, 27]. Changes of DTI metrics seem to be independent of the concomitant accumulation of focal lesions and reduction in brain volume [27]. Thus, the application of DTI in monitoring the evolution of MS-related tissue damage over time looks promising for possible future trials of neuroprotective therapies .
GM
DTI abnormalities have been demonstrated also in the GM of MS patients [3, 4, 25, 28, 29]. They tend to be more pronounced in patients with the progressive forms of the disease [4, 29, 30], especially in those with secondary progressive (SP) MS [29]. In patients with SPMS and primary progressive (PP) MS [31, 32], as well as in those with RRMS [33] and CIS [34], such DTI abnormalities worsen over time. The severity of GM damage has been correlated with the degree of cognitive impairment in mildly disabled RRMS patients [35] and has been found to predict accumulation of disability over a 5-year period in patients with PPMS [32].
Despite the presence of conflicting results, higher MD values have been detected in deep GM nuclei of MS patients, especially in the thalamus [36]. Such abnormalities are more pronounced in SPMS than in RRMS patients [36]. In another study, average NAWM MD and FA thalamic changes over 1 year follow-up were predictors of clinical deterioration after 5 years in PPMS patients [37].
Possible explanations for the observed changes in GM diffusivity include a retrograde degeneration of neurons caused by injury to WM fiber tracks [38] and the presence of otherwise undetected MS lesions in the GM. These hypotheses are supported by the observation that GM damage is only partially correlated with the extent of focal WM lesions and the severity of their intrinsic damage [3, 4].
Cortical Lesions
Pathologic and MRI studies have shown that cortical lesions (CLs) are frequent in MS, especially in the progressive forms of the disease. Two DTI studies have demonstrated that, compared to patients’ GM, intracortical MS lesions have increased FA values [39, 40], which might reflect an intralesional loss of dendrites, neuronal damage, and activation of microglial cells. One of these studies [40] also found that quantifying CL damage using DTI can help to distinguish the different MS clinical phenotypes, particularly benign (B) MS from SPMS patients (Fig. 16.3).
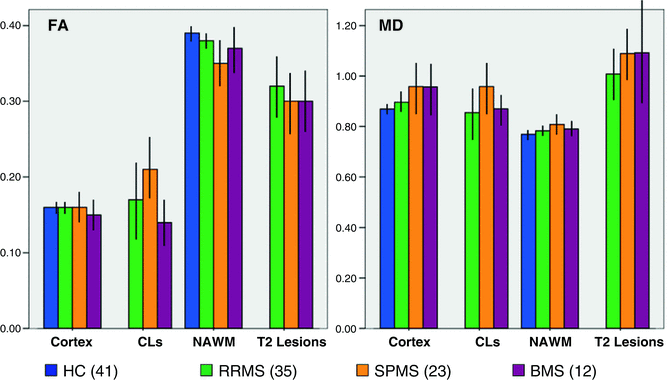

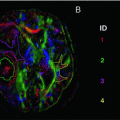
Fig. 16.3
Diffusion tensor imaging (DTI) values from cortical lesions (CLs), normal-appearing white matter (NAWM), and T2 lesions in multiple sclerosis (MS) patients with different disease clinical phenotypes and a group of healthy controls (HC). Compared to relapsing-remitting (RR) MS patients, those with secondary progressive (SP) MS had a higher T2 lesion volume and a more severe damage to the cortex, NAWM, T2 lesions, and CLs. With the exception of CLs, damage to all these compartments was similar in SPMS and benign (B) MS patients. Conversely, compared to SPMS, BMS patients had a lower fractional anisotropy (FA) and mean diffusivity (MD) of CLs. No difference was detected between RRMS and BMS patients. MD is expressed in units of mm2/s × 10−3, FA is dimensionless index. [Adapted from Filippi M, Preziosa P, et al. Microstructural MR imaging of cortical lesion in multiple sclerosis. Mult Scler. 2013;19(4):418–26. With permission from Sage Publications]
Optic Nerve
Early DTI studies of the optic nerve measured diffusivity in a few directions [41]. With the introduction of high-resolution fat- and CSF-suppressed zonal oblique multisection echo planar imaging (ZOOM-EPI) sequence [7, 42, 43], full DTI measurements from the optic nerve have been obtained.
It has been shown that in patients in the chronic phase following optic neuritis, MD of the diseased optic nerve is significantly higher than in either the fellow eye or those from healthy individuals [42]. DTI abnormalities were found to be correlated with abnormal visual evoked potentials (VEPs) latencies [42], loss of visual acuity [42, 44], and retinal nerve fiber layer thinning at optic coherence tomography, particularly at high contrast and in nerves previously affected by optic neuritis [44]. A multiparametric MRI study showed that, 4 years after a unilateral optic neuritis, decreased optic nerve FA and volume are factors independently associated with visual dysfunction [45].
Spinal Cord
With the development of sophisticated MR receiver coils and fast imaging techniques, it has been possible to obtain more reliable imaging of the spinal cord, which also allows the acquisition of quantitative data, such as DTI. Clark et al. [46] found higher MD values in spinal cord lesions of MS patients than in spinal cord from healthy controls. Abnormal DTI quantities from the cervical cord have been shown in patients with established MS [47, 48] and NMO [49] but not in those with CIS [50]. Cervical cord damage outside focal lesions is diffuse in SPMS, while it is more limited in BMS patients [51]. Several studies highlighted that brain and cord DTI metrics are independently associated with MS disability [27, 48, 49], thus calling for an aggregate use of these measures to improve the understanding of disease progression.
One longitudinal study which obtained DTI from relapse-onset MS patients at baseline and after a mean period of 2.4 years showed that baseline cord cross-sectional area and FA correlate with increased disability at follow-up [27].
Novel Strategies of Analysis
Tractography
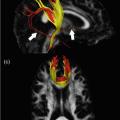
Thanks to the ability of DTI to depict anisotropic tissues [52] and to detect their intrinsic structural abnormalities, several approaches have been developed to investigate damage to selected WM tracts, with the ultimate goal of improving the correlation with clinical measures. DTI tractography can be used to segment clinically eloquent WM pathways involved in different functions, such as the corticospinal tract (CST) [10, 53–55], the corpus callosum (CC) [56], optic radiations (OR) [57], and many others [22, 58–60]. In line with studies performed using histogram or regions-of-interest analysis, these studies have detected higher MD and lower FA of WM tracts in MS patients compared to healthy controls. Moreover, tract-derived DTI metrics correlate with several measures of locomotor disability and cognitive impairment [10, 22, 53–62]. For instance, MD and FA values of the CST correlated with clinical measures of locomotor disability or the pyramidal functional system score of the expanded disability status scale (EDSS) more than T2 lesion volume and the overall extent of diffusivity changes of the brain [53–55]. CIS patients with motor impairment had increased MD and T2 lesion volume in the CST compared to patients without pyramidal symptoms [10].
Increased MD of the CC has been associated with MS cognitive dysfunction [54, 63]. Compared to cognitively unimpaired BMS patients, those with cognitive impairment had significantly higher NAWM MD of the CC [56]. By applying a random forest analysis to measures derived from DTI tractography, damage to “critical” WM tracts, such as the cingulum, was shown to contribute significantly to the cognitive impairment of MS patients [62].
Using DTI tractography of the ORs, one study showed that patients with optic neuritis had reduced connectivity values (as assessed by the number of reconstructed streamlines via tractography) in both ORs compared to healthy controls, suggesting the occurrence of transsynaptic degeneration secondary to optic nerve damage [57]. In another study, OR DTI abnormalities correlated with retinal injury, assessed using optical coherence tomography, and visual impairment [61].
Advances in DTI and tractography have spurred the development of brain neuro-connectivity techniques, which define and quantify anatomical links between remote brain regions by axonal fiber pathways [64]. The use of these approaches has revealed reduced network efficiency in the WM structural networks of MS patients [65], including those at the earliest stages of the disease [66].
Voxel-Wise Approaches
Voxel-wise approaches to the analysis of quantitative MRI data, such as voxel-based morphometry [67], hold promise for improving our ability to study the structural features of MS damage, since they assess the topographical distribution of brain damage at a voxel level. Despite voxel-based MRI studies of MS patients having mainly focused on measures of atrophy [68], this approach can also be used for the analysis of DTI data. A voxel-based study [69] showed that patients with RRMS and BMS differ in terms of topographical distribution of WM damage, while no between-group differences were found when the overall extent of WM diffusivity abnormalities was assessed.
By combining tractography and voxel-based analysis, another study evaluated damage to several WM tracts, in terms of focal lesions and NAWM, from the main clinical phenotypes of MS [22]. Compared to healthy controls, diffusivity abnormalities were found in PPMS and CIS patients. The progressive MS forms showed the most severe and distributed diffusivity abnormalities, whereas BMS patients had only a limited WM damage. These findings support the notion that the assessment of regional damage using DTI may be more rewarding than that of “global” brain damage in order to gain insight into the relation between clinical status and disease burden in MS.
Tract-based spatial statistics (TBSS) is a technique that allows voxel-wise analysis of multi-subject DTI data. Using such a technique, compared to healthy controls, MS patients had reduced FA values of several WM tracts, which were related to deficits of specific cognitive domains [70, 71]. A voxel-based method has been developed to obtain estimates of WM tract volumes using DTI [72]: an index of volume change is derived from the differences between an FA atlas based on the morphological characteristics of a reference population and an individual subject FA map. This approach has been successfully applied to assess the topographical distribution of age-related WM volume changes in healthy subjects [73] and may be helpful to improve our understanding of MS abnormalities.
Other Inflammatory Demyelinating Diseases
NMO
NMO is an inflammatory demyelinating condition clinically characterized by optic neuritis and transverse myelitis and by the presence of a serum autoantibody (NMO-IgG) found to specifically target aquaporin-4 (AQP4). A DTI study revealed more severe cervical cord damage in NMO than in MS patients [49]. The assessment of brain NAWM and GM damage in NMO patients gave conflicting results: some authors found an isolated involvement of the GM [74], while others described an involvement of several WM tracts [75], which was more severe in the ORs and CSTs [76].
ADEM
ADEM is classically defined as a monophasic dysimmune demyelinating disease, commonly affecting children, generally preceded by infections or vaccinations. A large effort has been invested in trying to define MRI parameters that are able to differentiate MS from ADEM. Using DTI, no abnormalities of the normal-appearing brain tissue and spinal cord have been detected in ADEM patients after the acute phase of the disease [77], whereas mild DTI abnormalities of the basal ganglia have been described [78].
Conclusions
Conventional MRI is limited by its lack of specificity to the heterogeneous pathological substrates of inflammatory and demyelinating diseases of the CNS. DTI has proved to be able to quantify the amount of intrinsic tissue damage of focal lesions and to detect more subtle abnormalities occurring in NAWM, GM, spinal cord, and optic nerve. DTI is contributing to improving the understanding of the mechanisms associated with the development of locomotor and cognitive impairment in patients with MS as well as the heterogeneity of the disease clinical phenotypes. The role of DTI in the diagnostic workup of patients with different demyelinating conditions deserves further and more extensive investigation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree