Mortality at less than 30 days
Mortality at more than 30 days
Reoperation rate
Complications
Adjustable gastric banding
0.07% (0.02–0.12)
0.21% (0.08–0.37)
7.01% (3.99–11.24)
7.80% (3.90–13.00)
Sleeve gastrectomy
0.29% (0.11–0.63)
0.34% (0.14–0.60)
2.96% (1.70–4.71)
8.90% (5.60–13.00)
Roux-en-Y gastric bypass
0.38% (0.22–0.59)
0.39% (0.01–0.86)
5.34% (4.48–6.48)
12.00% (7.30–17.00)
However, a mortality rate of 29.4% has been described in 2007 from the Italian Society for Obesity Surgery during a 10-year analysis [9]. The mortality risk has been related to different factors including type of surgery, prolonged operative time, comorbidities, and volume of activity [9].
Interventional radiology (IR) plays an important role in the multidisciplinary management of post-operative complications. A number of relevant IR techniques will be covered in more detail during the forthcoming chapter.
6.2 Post-operative Complications Following Bariatric Surgery
Each bariatric surgical procedure presents a different spectrum of complications. The most common procedures with their associated complications are as follows [11]:
6.3 Gastric Banding (GB)
GB includes procedures involving both adjustable and nonadjustable band placement.
Whilst the technique is in decline, many patients have implanted bands in situ.
Complications include:
Malpositioning of the gastric band: Commonly related to band slippage in the perigastric fat or distal stomach which can occur at various (early and late) periods of post-surgery.
Infection and gastric perforation (0.1–0.8% of patients): Band infection usually manifests in the early post-operative period with variable symptoms ranging from fever to severe abdominal pain and hypotension. Gastric perforation is uncommon and can lead to gastric obstruction/perforation.
Pouch dilatation occurs when there is overexpansion of the gastric pouch proximal to the band. This complication is usually due to band slippage/overinflation/overeating/stomal oedema; gastro-oesophageal reflux with vomiting may be the presenting symptoms.
Gastric band slippage and prolapse is defined as herniation of the distal stomach upward from below the band which may occur in an anterior or posterior direction. Slippage results in an abnormal band position with eccentric pouch dilatation and may lead to chronic stomal stenosis, which has been observed in 4–13% of patients. Slippage clinically presents with limited weight loss, severe gastro-oesophageal reflux, and nocturnal vomiting.
Intragastric erosions (occurring as 0.3–14% of patients in various series) are defined as partial or complete. Their aetiology may be secondary to small gastric wall injuries that can occur during band placement. Over-distension of the band can result in gastric wall ischaemia, band site infection, and inflammatory reactions. In addition, use of non-steroidal anti-inflammatory drugs may contribute to the degree of gastric erosions.
Oesophageal dysmotility and dilatation typically occur before oesophageal dilatation, but pre-existing insufficiency of the gastro-oesophageal sphincter may contribute. Other causes of oesophageal dilatation include insufficient change of dietary habit after the procedure, proximal pouch dilatation, and stomal narrowing.
Other delayed complications include disconnection of the band components, port site infection, and small bowel obstruction.
6.4 Sleeve Gastrectomy (SG)
– Gastric leak is the most common complication with an incidence of 1–10% of patients in published gastroplasty series [12]. The incidence can rise to 16–20% following repeat operative surgery [12]. Gastric leak has been defined by the UK Surgical Infection Study Group as “the leak of luminal contents from a surgical join between two hollow viscera” and is classified based on the timing of the leak during the post-operative period, namely, early (≤3 days after surgery), intermediate (≥4 and ≤7 days after surgery), and late (≤8 days after surgery) [13].
Classically leaks tend to appear between 5 and 6 days after surgery because of a lack of mural/anastomotic integrity. The typical location of gastric leak is the proximal third of the stomach, close to the gastro-oesophageal junction (85.7%), and less commonly occurs in the distal third (14.3%) [12]. Gastric leak management is still relatively empiric without accepted guidelines [13].
Collection/abscess is usually the result of gastric leak/fistulas and in many cases can be drained percutaneously under image guidance.
Haemorrhage and haematoma can be treated by percutaneous embolisation.
6.5 Roux-en-Y Gastric Bypass (RYGB)
RYBP includes both gastrojejunal anastomosis and enteroenteric anastomoses with both anastomoses susceptible to complications, which include:
Anastomotic leaks occur between 1.1 and 8.3% of patients during the early post-operative period and are managed in a similar fashion to leaks following the other main bariatric surgical procedures [12].
Gastrogastric fistulas : This rare complication, which may be the sequelae of a leak, results in a fistula between the proximal gastric pouch and excluded gastric remnant. The complication can lead to long-term problems of gastro-oesophageal reflux and stomal ulceration as well as patient weight gain.
Gastrojejunal anastomotic strictures are uncommon, and many are treated using endoscopy with balloon dilation.
Degradation of pouch restriction integrated often presents with a rapid passage of contrast material through a patulous anastomosis. The loss of the restrictive properties on the laparoscopic Roux-en-Y gastric bypass may cause the patient to feel insatiable and produce weight gain.
Small bowel obstruction is more common after laparoscopic gastric bypass than after open procedures with an incidence of up to approximately 8% [14]. The aetiology of small bowel obstruction may be due to a variety of causes including anastomotic leaks and narrowing, mural and mesenteric haematomas, post-operative adhesions, and internal hernias. Internal hernias may occur through defects in the small bowel mesentery and transverse mesocolon or through a potential space posterior to the Roux limb termed the Peterson space. Various simplified classification systems have been proposed to stratify the varied aetiologies.
Haemorrhage and haematoma commonly occur from the staple line. Endoscopic management using clips, adrenaline injection, and electrocautery can be used to manage bleeding from the proximal pouch, but haemorrhage from the distal pouch is more difficult to treat. Percutaneous embolisation techniques play a role when managing this complication.
Abscess is usually the result of intestinal perforation. These can be drained percutaneously under image guidance.
6.6 The Role of Interventional Radiology in the Management of Post-operative Surgical Complications
Interventional radiology (IR) plays an important role in the minimally invasive management of various post-operative bariatric surgical complications particularly when further surgical re-intervention increases the complication risk to the patient.
Since the advent of IR techniques, diagnostic imaging technology along with IR equipment has undergone rapid technological advances. In particular, developments in IR techniques and equipment have led to various safe and effective procedures. For example, manufacturing advances have resulted in a variety of catheters and guide wires with characteristics such as torsional strength, diameter, hydrophilic properties, and specific shape to the type of procedure undertaken.
In parallel, equipment used to guide interventional procedures has advanced with in particular the cross-sectional techniques of ultrasound (US) and computed tomography (CT) now widely available allowing the precise placement of interventional equipment. Multimodality imaging is also now routinely used during interventional procedures with more recent developments facilitating image fusion such as the overlay of 3D cross-sectional datasets with fluoroscopy. These advances have also been associated with the significant reduction in radiation exposure to both patients and staff.
The type of interventional procedure performed will depend on the specific post-operative complication, with IR techniques most commonly used to aspirate and drain collections, embolise/stent bleeding vessels, dilate anastomotic strictures, and more recently facilitate GI tract stent placement following leaks.
In addition, IR can play an important role in delayed complications including choledocholithiasis formation in patients following RYGB (Table 6.2).
Table 6.2
Demonstrating a temporal classification of complications
Early complications | Post-operative bleeding |
Acute anastomotic leak | |
Acute gastroenteric perforation/breakdown | |
Gastric band malposition/slippage | |
Late complications (more than 30 days post-operatively) | Delayed leak from anastomosis |
Delayed leak from gastroenteric perforation/breakdown | |
Choledocholithiasis | |
Stomal stenosis (at both anastomosis and gastric band locations) |
6.7 Aspiration/Drainage of Collections
Percutaneous drainage (PD) of post-operative collections is the first-line therapy for patients who do not have other indications for immediate surgery. This is particularly true for the post-operative bariatric patient with a well-contained collection.
Primary SG may have a leak rate of up to 9% with an increase in incidence of up to 13% following revision surgery.
Whilst authors have recommended immediate surgical re-intervention in order to close the anastomotic defect, other minimally invasive techniques may be used with Corona et al. reporting that PD has been the stand-alone procedure in 58% of patients in their unit with gastric leak after SG [15].
Whilst PD is generally safe and effective, procedures require careful planning in order to determine the optimum access pathway to a collection. In particular, a pathway should be direct and straightforward and avoid inadvertent injury to adjacent structures and organs. Pre-procedure planning and guidance are in most cases performed using either US or CT. The choice of modality depends on various parameters including collection characteristics, operator choice, and imaging availability.
US offers a number of advantages such as real-time imaging, no ionising radiation, accurate assessment of collection contents due to high-contrast resolution, and equipment mobility allowing procedures to be performed in the IR suite or at the patient’s bedside. The modality however is very operator dependent particularly in obese patients, and collections containing gas may be poorly visualised. In addition, enteric leaks that occur from anastomoses following gastric bypass procedures commonly present in anatomical locations adjacent/deep to gas-filled organs including the stomach, duodenum, small bowel, and colon making ultrasound guidance challenging or impossible. Therefore, whilst US can be used in large and superficial collections, CT plays an important role in this group of patients.
Despite the disadvantage of requiring ionising radiation, CT is widely used when feasible in the bariatric patient in order to plan the access pathway to a collection. The modality allows accurate assessment of the collection position, depth, and adjacent structures thus facilitating the calculation of the optimal angle/direction for the access pathway. The standard CT scanner however has a maximum allowed patient weight of around 160 kgs with a bore of 70 cms, thus restricting the use of the modality in this group of patients. In response to this drawback, a number of manufacturers have developed dedicated bariatric machines allowing up to 300 kgs bodyweight with an enlarged bore of 80 cms.
6.8 Equipment Choice
Needles: The majority of abdominal collections can be aspirated through an 18 gauge needle which offers approximately 1/20th the resistance to flow when compared to a 21/22 gauge needle. In addition, an 18 gauge needle is easier to visualise and control using both US and CT guidance as well as accepting a 0.038 inch guide wire.
The majority of 21/22 gauge needle systems however require insertion of an initial 0.018 inch guide wire followed by a coaxial dilator thus adding to the complexity of the procedure.
The typical manufacturing needle length is 15–20 cm, which may be inadequate to reach a deep collection in the bariatric patient. The use of a 55 cm Colapinto needle (Cook Incorporated, Bloomington, IN) will however allow access to most collections.
Catheters: Drainage catheters vary in size and design but invariably involve a locking pigtail design. Catheter effectiveness is based on the degree of kink resistance and internal diameter as well as choice of coating facilitating ease of placement.
Pigtail catheters are preferred because the design allows a reduced risk of accidental displacement. The choice of catheter size can be determined by the needle aspiration test, which dictates that if fluid can be easily aspirated through a 10 mL syringe (1 mL in 1 s) using an 18 gauge needle, then an 8.5 F catheter diameter will be effective.
Complex collections however may require a catheter size of up to 16 F.
6.9 Technique
The two standard techniques for percutaneous collection drainage are the trocar or “one-step” and Seldinger or “two-step” procedures.
Trocar technique : This uses a catheter mounted on a central trocar and stylet.
Following subcutaneous infiltration of local anaesthetic, a direct puncture with the mounted catheter is used to access the collection. The central stylet is then removed, and aspiration is performed to confirm correct catheter tip location. The catheter is then advanced over the trocar into the collection. The technique is only suitable for large and superficial collections.
Seldinger technique : The technique is more appropriate and in most cases much safer for use in bariatric patients. Following subcutaneous infiltration of local anaesthetic, an appropriate needle (usually 18 gauge) is introduced into the collection under image guidance. After successful aspiration, a guide wire is then inserted through the needle into the collection, and following needle removal, the tract is dilated over the wire to the required diameter prior to wire-guided placement of the catheter. If a smaller needle diameter is initially used to puncture the collection, then either further guide wire exchanges can be used to upsize the tract or a second 18 gauge needle can be introduced adjacent to the initial needle (Fig. 6.1).


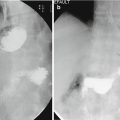
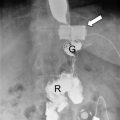
Fig. 6.1
CT-guided percutaneous drain placement using the Seldinger technique : (a) Non-contrast CT scan with oral contrast (Gastrografin), confirming a leak and perigastric collection. (b) CT-guided puncture of the collection using an 18 gauge coaxial needle. (c) Subsequent fluoroscopic image confirming position of a 12 French Malecot (Cook Medical) drain within the collection
Both techniques require appropriate final catheter fixation to the skin with a range of devices available. Importantly an adequate specimen must be sent to microbiology and free drainage of collection contents confirmed into the catheter bag.
Collections containing viscid contents require regular flushing using 10–20 mL of saline once or twice daily in order to maintain catheter patency.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree