Criteria
RECIST 1.0
RECIST 1.1
Overall tumor burden assessment
Measuring maximum of ten lesions, with up to five lesions per organ
Measuring maximum of five lesions, with up to two lesions per organ
Minimum size of measurable lesions
10 mm on spiral CT or 20 mm on non-spiral CT
CT: 10 mm (when slice thickness is 5 mm or smaller); or 2× slice thickness (when slice thickness is larger than 5 mm)
Lymph nodes
Not mentioned
Target lesions: ≥15 mm, nontarget lesions: ≥10–<15 mm, non-pathological lesions: <10 mm (based on short axis diameter on CT)
Measurability
Not specified
Special notes on bone lesions and cysts
CR of target lymph nodes
Not specified
CR: short axis diameter of LN <10 mm (therefore, SLD may not be 0 mm in CR)
PD of target lesions
PD: ≥20 % increase over smallest SLD
PD: both ≥20 % increase over smallest SLD and absolute ≥5 mm increase of target lesions
PD of nontarget lesions
Increase in size of previous nontarget lesions is considered PD, regardless of the status of target lesions
Specification: increase of previous nontarget lesions is PD only if the increase is representative of change in overall tumor burden
New lesion
Not specified
Detailed description for lesions considered new
Overall response
No special notes
Notes on residual tissue, including lymph nodes
Imaging appendix
Contains some specifications for imaging
Updated with detailed guidance on use of MRI, FDG-PET(/CT), and other practical issues
Therefore, the HCC panel of European Association for the Study of the Liver (EASL) established the EASL criteria in 2001. EASL criteria recommended that viable tumor burden, represented by arterially enhancing areas within the tumor on dynamic imaging modality after treatment, should be assessed. The EASL criteria have shown to be superior in assessing treatment responses and predicting survival outcomes in patients with HCC compared with the WHO and RECIST guidelines. Recently, mRECIST (modified RECIST) was released in 2010 to formally amend RECIST. In the mRECIST guideline, the concept of viable tumor, which shows arterial enhancement on contrast-enhanced radiologic imaging techniques, was adapted. Like the EASL criteria, the mRECIST criteria also showed superior efficacy in assessing treatment responses and predicting survival outcomes in patients with HCC compared with conventional size-based criteria (WHO and RECIST guidelines). Compared with the EASL criteria, the mRECIST is simpler to assess therapeutic response of HCC after treatment, since the mRECIST is based on one-dimensional measurement of up to two measurable enhancing target lesions in the liver, whereas EASL criteria is based on two-dimensional measurement of all measurable target lesions. According to recent studies, the mRECIST was equivalent to or better than EASL in predicting survival in patients with HCC after chemoembolization.
9.3 Modified RECIST (mRECIST)
Table 9.2 summarizes the differences of assessing therapeutic response for HCC between conventional RECIST and mRECIST. The size of target lesion is measured by the longest diameter of the tumor in conventional RECIST criteria, regardless of its enhancement. In mRECIST, it is measured by the longest diameter of arterial enhancing viable tumor. Other than that, the basic concept is similar. Since non-enhancing areas within a tumor is not considered as viable in mRECIST, measuring viable tumor diameter should not include any major intervening non-enhancing areas such as areas of necrosis. Criteria to be a target lesion in mRECIST are as follows: (A) at least one dimension of the lesion is 1 cm or larger, (B) the lesion is suitable for repeated measurement, and (C) the lesion shows intratumoral arterial enhancement on contrast-enhanced computed tomography or magnetic resonance imaging. In mRECIST for HCC, like conventional RECIST, overall response assessment of a patient is based on combined assessment of target, nontarget, and new lesions (Table 9.3).
Table 9.2
Assessment of target lesion response for HCC according to conventional RECIST and mRECIST
RECIST | mRECIST | |
|---|---|---|
Complete response | Disappearance of all target lesions | Disappearance of all intratumoral arterial enhancement in every target lesions |
Partial response | 30 % or greater decrease in the sum of the largest diameters of target lesions, with the baseline sum of the diameters of target lesions as reference | 30 % or greater decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, with the baseline sum of the diameters of target lesions as reference |
Stable disease | Any cases classified neither as either partial response nor progressive disease | Any cases classified neither as partial response nor progressive disease |
Progressive disease | 20 % or greater increase in the sum of the diameters of target lesions, with the smallest sum of the diameters of target lesions recorded since treatment started as reference | 20 % or greater increase in the sum of the diameters of viable (enhancing) target lesions, with the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started as reference |
Table 9.3
Overall response assessment in mRECIST
Target lesions | Nontarget lesions | New lesions | Overall response |
|---|---|---|---|
CR | CR | No | CR |
CR | IR/SD | No | PR |
PR | Non-PD | No | PR |
SD | Non-PD | No | SD |
PD | Any | Yes or no | PD |
Any | PD | Yes or no | PD |
Any | Any | Yes | PD |
9.4 Summary
1.
In both conventional RECIST and mRECIST, overall response assessment of a patient is a result of the combined assessment of target lesions, nontarget lesions, and new lesions.
2.
Size-based assessment of therapeutic response such as RECIST or WHO criteria can be misleading when applied to locoregional therapies or molecular-targeted therapies of HCC.
3.
In conventional RECIST criteria, the size of target lesion is measured by longest diameter of target lesion, regardless of its enhancement, whereas it is measured by longest diameter of arterial enhancing viable tumor in mRECIST.
4.
Since non-enhancing area within tumor is not considered viable tumor in mRECIST, the measurement of the viable tumor diameter should not include any major intervening areas of non-enhancing area suggesting necrosis.
9.5 Illustrations: Therapeutic Response Evaluation of HCC
9.5.1 Application of RECIST 1.1 for Assessing Therapeutic Response of Hepatocellular Carcinoma (HCC)
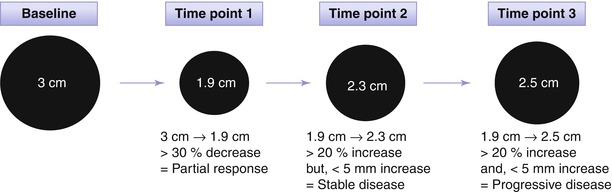
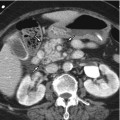
Fig. 9.1
Application of RECIST 1.1 for assessing therapeutic response of hepatocellular carcinoma (HCC). Baseline sum of the longest diameter (SLD) of target lesions is 3.0 cm. At time point 1 after treatment, SLD decreases to 1.9 cm and the decrease in SLD is larger than 30 %, thus partial response is declared. At time point 2, SLD increases to 2.3 cm from the lowest SLD (1.9 cm). Although the change in SLD is larger than 20 %, it should be determined stable disease since the increase in SLD is less than 5 mm. At time point 3, the SLD reached to 2.5 cm and now the increase in SLD from the lowest SLD is larger than 5 mm. Therefore, therapeutic response is progressive disease at time point 3
9.5.2 Difference of RECIST and mRECIST for Assessing Therapeutic Response of Hepatocellular Carcinoma (HCC)
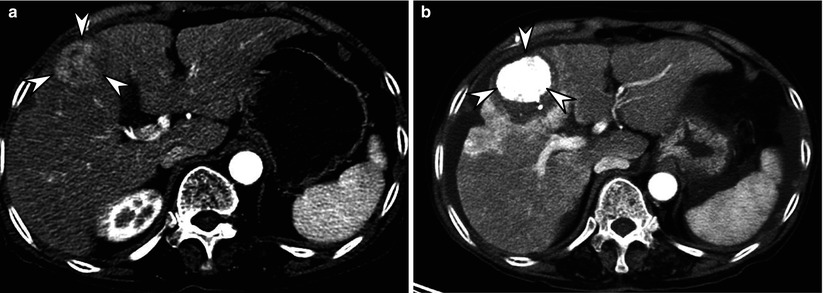
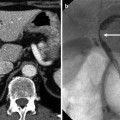
Fig. 9.2
Difference of RECIST and mRECIST for assessing therapeutic response of hepatocellular carcinoma (HCC). (a) Before treatment, arterial enhancing 3.3-cm-sized HCC (arrowheads) is seen in right lobe of liver. (b) On an arterial phase CT image obtained immediately after combined chemoembolization and RFA, compact iodized oil uptake (arrowheads) in the tumor is noted. In addition, there is unenhanced area surrounding the entire tumor, indicating sufficient ablative margin. The size of tumor is nearly unchanged after treatment. Therefore, this can be considered stable disease according to RECIST criteria. However, this case can be assessed as complete response according to mRECIST criteria due to absence of any enhancing viable tumor
9.5.3 Measurement Methods of Tumor Size in RECIST and mRECIST for Assessing Therapeutic Response of Hepatocellular Carcinoma (HCC)
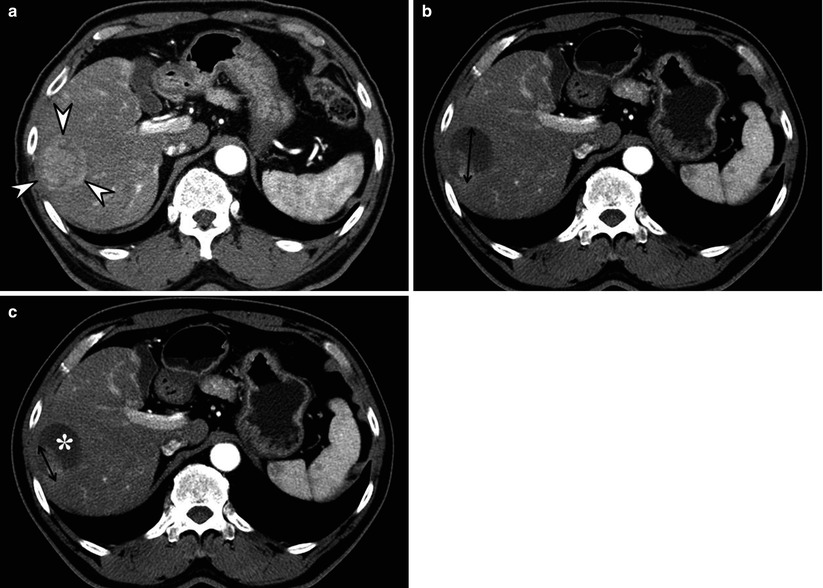
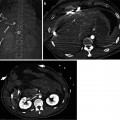
Fig. 9.3
Measurement methods of tumor size in RECIST and mRECIST for assessing therapeutic response of hepatocellular carcinoma (HCC). (a) Before initiating treatment, arterial enhancing HCC (arrowheads) is seen in right lobe of liver. (b) On an arterial phase CT image obtained 1 month after doxorubicin drug-eluting beads transcatheter arterial chemoembolization (DEB TACE), degree of enhancement and the size of enhancing area within the tumor decreases, suggestive of tumor necrosis. According to conventional RECIST, longest diameter of overall tumor regardless of tumor enhancement is measured. (c) According to mRECIST, longest diameter of only enhancing portion precluding intratumoral necrotic area (asterisk) is measured
9.5.4 Measurement Methods of Tumor Size in mRECIST for Assessing Therapeutic Response of Hepatocellular Carcinoma (HCC)
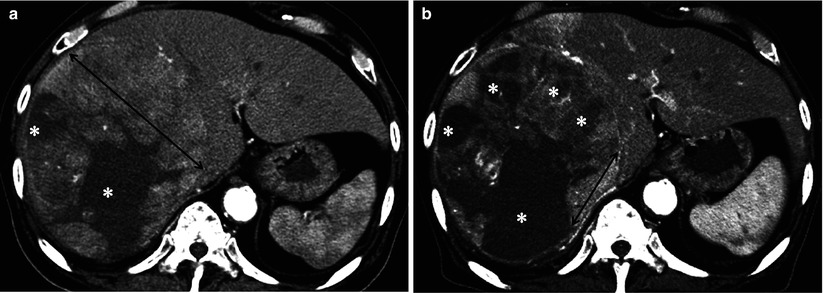
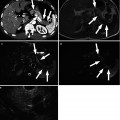
Fig. 9.4
Measurement methods of tumor size in mRECIST for assessing therapeutic response of hepatocellular carcinoma (HCC). (a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree