Study
Year of publication
TNM 2002
Number of patients
Androgen suppression therapy
External irradiation
Effect of overall survival
Androgen suppression + radiotherapy versus radiotherapy alone
(a) Adjuuvant (+/– concomittant) androgen suppression
EORTC 22863 Bolla et al. (2010)
2002
T1–2 poorly differentiated M0 or T3–4N0-1 M0
415
LHRHa for 3 years
70 Gy RT
Significant benefit for combined treatment (HR = 0.51, 95 % CI: 0.36–0.73, p = 0.0002)
RTOG 85-31 Pilepich et al. (2005)
2005
T3 or N1M0
977
Orchiecotomy or LHRHa
65–70 Gy RT
Significant benefit for combined treatment (p = 0.002) seems mostly caused by patients with Gleason score 7–10
Granfors et al. (2006)
2006
T3N0–1M0
91
Orchiectomy
65 Gy RT
Significant benefit (p = 0.02), mainly caused by lymph node positive tumors
D’Amico et al. (2008a)
2008
T2N0M0 (localized unfavorable risk)
206
LHRHa + flut. 6 months
70 Gy 3D-CRT
Significant benefit (HR = 0.55, 95 % CI: 0.34–0.90, p = 0.01) that may pertain only to men with no or minimal comorbidity
(b) Neiadjuvant and concomittant androgen suppression
TROG 96-01 Denham et al. (2005)
2005
T2b–T4 N0M0
802
Goserelin + flutamide 3 or 6 months before + concomitant
66 Gy
No significant difference in overall survival reported. Benefit in prostate-cancer-specific survival (HR = 0.56 [0.32–0.98], p = 0.04)
RTOG 94-13 Lawton et al. (2007)
2007
T1c–T4N0-1M0
1,292
2 months neoadjuvant + concomitant versus 4 months adjuvant
Whole pelvic RT versus prostate only 70.2 Gy
No significant difference in neoadjuvant + concomitant versus adjuvant ADT groups (interaction suspected)
RTOG 86-10 Roach et. al. (2008)
2008
T2–4N0-2
456
Goserelin + flutamide 2 months before + concomitant
65–70 Gy
No significant difference at 10 years
Short-versus long-term androgen suppression adjuvant (± concomittant) to radiotherapy
RTOG 92-02 Horwitz et al. (2010)
2008
T2c–4N0-1M0
1,554
LHRHa 2 years adjuvant after 4 months neoadjuvant
65–70 Gy RT
p = 0.73 overall. Significant benefit (p = 0.044) in subset with Gleason 8–10
EORTC 22961 Bolla et al. (2009)
2009
T1c–T2abN1M0 T2c–4N0-1M0
970
LHRHa 6 months versus 3 years
70 Gy 3D-CRT
Better result with 3-year treatment than with 6 months (+3.8 % survival at 5 year)
Androgen suppression therapy + radiotherapy versus androgen suppression alone
SPCGF-7/SFUO-3 Widmark et al. (2009)
2009
T1b-T2 Grade 2–3 T3N0M0
880
LHRHa 3 months + continuous flutamide
70 Gy RT versus no RT
Significantly better survival with combined treatment (HR = 0.68.95 % CI: 0.52–0.89, p = 0.004)
NCIC CTG PR.3 MRC PRO7/SWOG Warde et al. (2010)
2010 ASCO
T3-4 N0M0
1,205
Continuous LHRHa
60–65 Gy RT versus no RT
Significant benefit in favor of combined treatment (HR = 0.77.95 % CI 0.61–0.98, p = 0.033)
French study Mottet et al. (2010)
2010 ASCO
T3–4 N0M0
273
LHRHa for 3 years
70 Gy 3D-RT versus no RT
Significant reduction of clinical progression. Effect on overall survival not reported
3.1 Locally Advanced Prostate Cancer
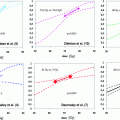
The main trials showing a benefit on overall survival were launched by the Radiation Therapy Oncology Group (RTOG) and the RTOG of the European Organization on Treatment and Research on Cancer (EORTC). Devoted to T3-4 N0-X M0 patients and sometimes bulky T2 patients, these trials deal with an agonist analog of LHRH. Two trials were carried out before the introduction of LHRH agonists using conventional modalities of castration. One of these trials, conducted at the MD Anderson Cancer Centre on a cohort of T3 NX M0 patients (n = 78) treated with pelvic RT ± diethylstilbestrol (DES, 5 mg), has shown a striking difference in 15-year disease-free survival in favor of the combined treatment, that did not translate into an improvement of overall survival (Zagars et al. 1988). The other trial, launched by the Medical Research Council (Fellows et al. 1992), focused on 277 T2-4 NX M0 cases treated by castration (n = 90), RT (n = 88) or combined treatment (n = 99): irradiation was left to the discretion of each center. Results of that trial showed that orchiectomy delayed the onset of distant metastases, and RT or orchiectomy proved equally effective in controlling local disease.
3.1.1 Concomitant and Long-Term LHRH Adjuvant Androgen Deprivation Therapy
The EORTC trial 22863 was the first to show a gain in overall survival ( Bolla et al. 1997). It recruited 415 patients classified as T1-2 NO histological grade 3 WHO or T3-4 N0 M0 to compare RT with concomitant and adjuvant ADT to RT alone, and a deferred ADT in case of relapse; 82 % of the patients were T3, 10 % T4, and 89 % N0. The hormone treatment was oral cyproterone acetate, 50 mg 3 times daily for 1 month, beginning 1 week before the start of RT and subcutaneous injection of Zoladex® 3.6 mg every 4 weeks for 3 years starting on the first day of RT. The pelvic target volume received 50 Gy and the prostatic target volume 20 Gy. With a median follow-up of 66 months, there was a significant difference in overall survival, 78 % in favor of the combination versus 62 % for RT alone (p = 0.001) (Bolla et al. 2002). The 10-year results (median follow-up of 9.1 years), confirm that the addition of HT increased the clinical disease-free survival from 22.7 to 47.7 % (P < 0.0001), distant progression free survival (PFS) from 30.2 to 51.0 % (P < 0.0001), and overall survival from 39.8 to 58.1 % (P = 0.0004). The 10-year PCa mortality was 30.4 % with RT alone and 10.3 % with long-term ADT combined with RT (P < 0.001) (Bolla et al. 2010a) and no significant difference in cardiovascular mortality was noted between treatment groups.
3.1.2 Long-Term LHRH Adjuvant Androgen Deprivation Therapy
The RTOG Trial 85-31 was designed to evaluate the effectiveness of indefinite Zoladex® alone after RT; 977 patients with stages T3–T4 M0 with or without lymph node involvement, or pT3 after radical prostatectomy in the event of capsule invasion, positive margins, or seminal vesicle involvement were included. Monthly administration of Zoladex® was started during the last week of RT and was continued indefinitely or until relapse (arm 1) or started at relapse (arm 2); no antiandrogen was given at the very start of Zoladex® to inhibit the initial rise of LH and then of testosterone. Fifteen percent of patients had undergone radical prostatectomy in arm 1 and 14 % in arm 2, and 29 and 26 % had lymph node involvement, respectively. The pelvic target volume received 45 Gy and the prostate target volume 65–70 Gy. Patients with a pT3 tumor received 60–65 Gy to the postoperative target volume. The combined approach has been associated with all 8-year efficacy endpoints except overall survival (49 % vs. 47 % (p = 0.36)); subset analysis by Gleason score, revealed a significant overall survival (p = 0.036) in favor of the adjuvant HT arm for centrally reviewed Gleason 8–10 patients who had not previously undergone prostatectomy (Lawton et al. 2001). In the updated results of the trial after a median follow-up time of 7.6 years, statistical significances were reached in favor of the adjuvant HT arm for 10-year overall survival (49 % vs 39 %, p < 0.002), 10-year incidence of distant metastases (24 % vs 39 %, p < 0.001), and disease specific mortality (16 % vs 22 %, p = 0.005) (Pilepich et al. 2005).
A subset analysis of the RTOG 85-13 trial evaluated 173 patients with biopsy proven pN1 lymph nodes of whom 98 received RT plus adjuvant HT; with a median follow-up of 6.5 years multivariate analysis revealed that the combined approach had a statistical impact on all endpoints: overall survival (p = 0.03), disease-specific failure (p = 0.014), metastatic failure (p < 0.0005), and biochemical control (p < 0.0001) (Lawton et al. 1997). These data are in keeping with those of Gransfors et al. (1998) who compared for T1-4 pN0-3 M0 patients, the combination of orchiectomy and RT (n = 45) to RT alone and androgen ablation deferred at clinical disease progression (n = 46). The study was prematurely closed due to an insufficient accrual, and after a median follow-up of 9.3 years there was a significant difference in overall survival (p = 0.02) and progression free survival (p = 0.005) in favor of the combined arm; this difference was mainly caused by lymph node positive tumors. In conclusion, patients with pathologically or clinically involved pelvic lymph nodes should be considered for RT plus immediate long-term HT (level of evidence 2b).
3.1.3 Long-Term Anti-Androgen Adjuvant Monotherapy
The Early PCa Program consisting of 3 randomized, double-blind placebo-controlled trials included 1370 patients with T1-4, any N M0 PCa. A nonsteroidal antiandrogen—bicalutamide (Casodex®) 150 mg/day orally was given as immediate adjuvant to RT during 2 years (trial 23), 5 years (trial 24), or until progression (trial 25), as an alternative to castration due to the potential benefits in term of sexual interest, physical capacity, and maintenance of bone mineral density. At a median follow-up of 5.3 years (Tyrell et al. 2005) bicalutamide 150 mg significantly reduced the risk of disease progression (p = 0.003) in patients with locally advanced PCa (n = 305).
3.1.4 Neoadjuvant and Concomitant Short-Term Androgen Deprivation Therapy
The RTOG trial 86-10 was designed to test the potential value of a combined ADT prior (2 months) and during RT (2 months) with respect to RT alone, or at relapse: 471 patients with bulky (5 × 5 cm) tumors (T2-4) with or without regional lymph node involvement were included: 7 % had a positive nodal status in the combined treatment arm versus 9 % in the RT alone arm. Thirty percent of patients had a T2 tumor, and 70 % were classified as T3-4. Hormonal treatment consisted of oral flutamide (250 mg 3 × day) and a subcutaneous injection of Zoladex® 3.6 mg every 4 weeks (Pilepich et al. 2001). The pelvis received 45 Gy and the prostate target volume 65–70 Gy. At 8 years, ADT has been associated with all efficacy endpoints except overall survival, but subset analysis demonstrated that a significant enhancement in overall survival was seen in patients with Gleason score 2–6: 70 % versus 52 %; p = 0.015. These results were maintained at 10-year with a significant difference in disease specific mortality (23 % vs. 36 %; p = 0.01), distant metastases (35 % vs. 47 %; p = 0.006), disease-free survival (11 % vs. 3 %; p < 0.0001), but no difference in 10-year overall survival (43 % vs. 34 %; p = 0.12) (Roach et al. 2008).
The Trans-Tasman Radiation Oncology Group 96.01 trial has included 818 men randomly assigned to RT alone (66 Gy/33 fractions) (Scardino et al. 2003; Denham et al. 2005) 3 months’ androgen deprivation with goserelin and flutamide starting 2 months before RT; or 6 months’ ADT with the same regimen starting 5 months before RT. After a median follow-up of 10.6 years, compared with patients assigned RT alone, those assigned to 3 months’ ADT had a decrease cumulative incidence of PSA progression (p = 0.003), and local progression (p = 0.0005), and event-free survival (p = 0.0001). Six months’ ADT-reduced PSA progression (p < 0.0001) and local progression (p = 0.0001) and led to a greater improvement in event-free survival (p < 0.0001); moreover 6 months’ ADT decreased distant progression (p = 0.001), cancer-specific mortality (p = 0.0008), and all-cause mortality (p = 0.0008) compared with RT alone (Denham et al. 2011).
These two trials suggest that the significant impact of HT on disease-specific survival is certainly due to the concomitant component of HT during RT. In the trial reported by Crook’s et al. (2004), 378 patients were randomized between 3 months or 8 months neoadjuvant combined ADT with flutamide and gosereline before RT (66 Gy): with a median follow-up of 44 months there was no impact on biochemical control or survival.
Nevertheless, starting ADT 2 or 3 months before RT (and continuing it during RT) may be useful to decrease the tumor volume of high risk PCa and to improve the dose to organs at risk.
3.1.5 Short-Term Neoadjuvant Versus Short-Term Adjuvant Combined Androgen Deprivation Therapy with Whole Pelvis or Prostate Only Radiotherapy
RTOG 94-13 study is a four arm trial devoted to 1323 patients T1c-4 N0 M0 PSA <100 ng with an estimated risk of lymph node involvement >15 % based on the equation: risk of positive nodes = (2/3) PSA + ((GS)-6) × 10). The first randomization is done between neoadjuvant concurrent ADT (NCADT)—2 months before and 2 months during RT—and 4-month adjuvant hormone therapy (AADT) after RT; the second randomization took place between whole pelvis radiotherapy (WPRT) followed by a boost to the prostate or prostate only radiotherapy (PORT). WPRT plus NCADT improved the 4-year progression free survival (61 %) compared with PORT + NCADT (45 %), PORT + AADT (49 %) and WPRT + AADT (47 %) (p = 0.008) and there was no advantage to WPRT over PORT without neoadjuvant ADT (Roach et al. 2003). With longer follow-up progression free survival and biochemical failure (Phoenix definition) continue to favor the WPRT arm (p = 0.034 and 0.0098, respectively) but we await the major secondary endpoints, cause-specific and overall survival, since not enough events had occurred (Lawton et al. 2007).
3.1.6 Long-Term Androgen Deprivation Therapy Alone is Inferior to Long-Term Androgen Deprivation Therapy Plus Radiation Therapy
The above-mentioned studies have shown the efficacy of hormonal treatment combined with RT, but the impact of LTADT alone was not assessed so far. The SPCG-7/SFUO-3 trial has included 875 patients T1b–T2, G2–G3, or T3 any WHO histological grade (1–3) (78 % of T3) with baseline PSA < 70 ng/ml; patients were randomly allocated to endocrine treatment alone with 3 months of total androgen blockade followed by continuous flutamide (n = 439 patients), or to the same endocrine treatment combined with RT (n = 436 patients). After a median follow-up of 7.6 years, the cumulative incidence at 10 years for PCa specific mortality was 23.9 % in the endocrine alone group and 11.9 % in the endocrine plus RT group for a relative risk of 0.44 (0.30–0.66); the cumulative incidence for overall mortality was 39.4 % and 29.6 % with a relative risk of 0.68 (0.52–0.89) (Widmark et al. 2009). In conclusion, in patients with locally advanced or high risk localized PCa, the combination of RT to HT halved the 10-year PCa specific mortality and decreased overall mortality with fully acceptable risk of side-effects, compared to HT alone.
Protocol NCIC CTG PR-3/MRC PR07/SWOG included 1205 patients with T3-4 (n = 1057) or T2, PSA > 40 ng/ml (n = 119), or T2, PSA > 20 ng and Gleason > 8 (n = 25) and N0-X M0 PCa who were randomized to lifelong ADT (bilateral orchidectomy or LHRH agonist) with or without RT (65–70 Gy to prostate ± 45 Gy to pelvic lymph nodes). With a median follow-up of 6 years, the addition of RT to ADT significantly reduced the risk of death (p = 0.033) and the risk of specific death (p = 0.001) (Warde et al. 2010).
The Mottet trial included 273 patients with locally advanced PCa T3-4 or pT3 N0 M0 randomly assigned to lifelong ADT by LHRH agonist (leuproreline) with or without RT (70 Gy to prostate plus 48 ± 2 Gy to pelvic lymph nodes). With a median follow-up of 67 months, there was a significant improvement of the 5-year disease-free survival (p < 0.001), metastatic disease-free survival (p < 0.018) loco-regional progression free survival (p < 0.0002), but the effect on overall survival was not reported (Mottet et al. 2010).
3.1.7 Short-Term Androgen Deprivation Therapy is Inferior to Long-Term Androgen Deprivation
The aim of RTOG protocol 92-02 devoted to 1554 patients classified T2c-4N0, was to investigate the value of a long-term adjuvant ADT (LTADT) after a short-term ADT (STADT). All patients received 2 months of CADT with Zoladex® and flutamide before RT, followed during RT; a radiation dose of 65–70 Gy was given to the prostate. Patients were randomly assigned to receive no additional therapy or 24 months of Zoladex®. Compared with the STADT, the LTADT arm showed significant improvement in all efficacy endpoints except 5-year overall survival; in a subset of patients Gleason scores 8–10, the LTADT arm had significantly better overall survival: 81 % versus 70.7 % (p = 0.04) (Hanks et al. 2003). The 10-year results confirmed significant benefits in all 10-year efficacy endpoints terms except overall survival (p = 0.35); in a subset analysis the overall survival benefit was limited to patients with Gleason score 8–10 (p = 0.006) (Horwitz et al. 2008).
EORTC (22863) and RTOG (85–31) trials have demonstrated that LTADT (>2 years) is recommended for high risk PCa (level I evidence) but they do not determine the optimal duration of hormonal treatment combined with external beam RT. That is why the EORTC equivalence trial 22961 randomly assigned patients who had received 3D-CRT plus 6 months of ADT in two groups: one to receive no further treatment (STADT) and the other to receive 2.5 years of further treatment (LTADT) with a LHRH agonist, triptoreline, Decapeptyl 11.25 mg®. An outcome of noninferiority of STADT as compared to LTADT required a hazard ratio of more than 1.35 for overall survival, with a one-sided alpha level of 0.05. An interim analysis showed futility, and the results are presented with an adjusted one-sided alpha level of 0.0429. 970 patients were randomized: 483 STADT and 487 LTADT. At a median follow-up of 6.4 years, the 5-year overall survival shows 84.8 % for the LTADT arm and 81 % for the STADT arm with an estimated hazard ratio of 1.42 (P = 0.008). The 5-year clinical progression free survival was 80.5 % for the LTADT arm and 68.7 % for STADT arm (P < 0.0001). The 5-year biochemical progression free survival was 77.7 % on the LTADT arm versus. 56.8 %on the STAD arm P < 0.0001. In conclusion, the combination of RT plus 6 months of ADT provides inferior survival as compared with RT plus 3 years of ADT (Bolla et al. 2009).
Additional support can be found in a retrospective analysis assessing combined HT with RT (median follow-up >45 months) which showed that long-term ADT (median duration 25.6 months) improves 5-year overall survival (87.5 %) with respect to short-term ADT (75 %) (p = 0.009) in patients with a PSA level >20 ng/ml, irrespective of Gleason score and T stage (Berthelet et al. 2005).
3.2 Intermediate and High Risk Localized Prostate Cancer
3.2.1 6-Month Neoadjuvant and Concomitant Short-Term Androgen Deprivation Therapy
The Boston group published a small trial concerning 206 men with localized (T1b-T2b N0-X M0) but unfavorable-risk PCa (baseline PSA ≥ 10 ng/ml and ≤40 ng or a Gleason score of at least 7); patients were randomized to receive RT alone (70 Gy 3D-CRT) or RT plus 6 months of ADT; low risk patients were ineligible unless they had radiologic evidence of extracapsular extension or seminal vesicle invasion. After a median follow-up of 4.5 years, patients who received 3D-CRT plus ADT had a higher survival (p = 0.04) and a lower cancer-specific mortality (p = 0.02) (D’Amico et al. 2004). With a median follow-up of 7.6 years, overall survival was higher for men who were randomized RT and ADT compared with RT: 74 % versus 61 % (p = 0.01), but the survival benefit varies according comorbidity: among the 49 patients with moderate or severe comorbidity, the 8-year overall survival was 25 % for those randomized to RT and ADT as compared to 54 % for those with RT (p = 0.08) (D’Amico et al. 2008a).
3.2.2 4-Month Neoadjuvant and Concomitant Short-Term Androgen Deprivation Therapy
In RTOG trial 94-08 (Jones et al. 2011) which has accrued 1979 patients with T1b–T2b localized PCa, a stratification was done with PSA (≤ 20 ng/ml), histological grade and nodal status. Patients were randomized between neodjuvant CADT, 2 months before conventional RT (66.6 Gy) and 2 months during RT versus RT alone. The 10-year overall survival was 62 % for the combined approach as compared with 57 % (p = 0.03) among patients receiving RT alone. Biochemical failure, distant metastases, and the rate of positive findings on repeat prostate biopsy at 2 years were significantly improved with RT plus STADT, but the gains in overall survival and reductions in disease specific mortality were mainly limited to men in the intermediate risk subgroup.
In conclusion, 6-month of neoadjuvant and concomitant CADT combined with 3D-CRT (70 Gy) improved overall survival in men with intermediate or poor-risk localized PCa without moderate or severe comorbidity, meanwhile a conventional RT (66.6 Gy) plus 4-month of CADT improves overall survival only in men with intermediate localized PCa.
4 New Trends
4.1 4–6 Month Combined Androgen Deprivation Therapy Versus 6-Month LHRH Analog
The rationale of using an anti-androgen in association with an LHRH agonist is: (1) to block the androgens of adrenal origin, which are left free to continue to stimulate PCa (Labrie et al. 1993); (2) to block the androgen receptors (AR) to prevent the so-called “flare” that can result due to the surge in testosterone resulting from the use of LHRH agonist; and (3) to contribute independent anti-tumor activity. To know the optimal duration of combined androgen blockade in high risk patients would require a large phase III randomized trial. Since a meta-analysis of 27 randomized trials devoted to advanced PCa has shown that the addition of an anti-androgen to androgen deprivation, improved the 5-year survival by about 2 or 3 %, with a range of uncertainty between 0 and 5 %, it is unlikely that the effect would be very large (PCTCG 2000); but a small effect in patients with metastatic disease might be larger in men with high risk localized PCa analogous to the benefits of adjuvant 5-Fu chemotherapy for regional as compared to metastatic disease (Bauer and Spitz 1998; Colucci et al. 1999; Focan et al. 2000). Considering the positive impact of 4-month (Jones et al. 2011) or 6-month (D’Amico et al. 2008a) CADT on the overall survival of intermediate and high risk localized PCa and the positive impact of 6-month CADT on locally advanced PCa (Denham et al. 2011), CADT has to be preferred to LHRH agonists alone. Moreover, it has been shown that men with localized but unfavorable-risk PCa who were treated with RT and 6-month of planned combined ADT appear to have an increased risk of recurrence when treated with less than as compared with 6 months of the antiandrogen; recurrence risk was significantly decreased (p = 0.001) with each additional month of antiandrogen use after analysis adjustment for prognostic factors (D’Amico et al. 2008b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree