Fig. 1
Examples of different types of focal therapy. Ultra-focal therapy refers to treating a a specific target lesion or b multiple target lesions with a margin. Hemi-focal therapy refers to treating a half the gland with the urethra serving as midline or b treating the peripheral zone only. Focused therapy refers to differentially treating parts of the prostate gland. Examples of this include a full dose to the prostate gland but with sparing of the urethra and neurovascular bundles, b full dose to the prostate gland and a boost to a target lesion, and c full dose to a target lesion but less than full dose to the whole prostate gland. Blue circle = prostate gland, red filled circle = urethra, red open circles = treatment target, orange rectangle = neurovascular bundle
Irrespective of the approach (ultra-focal, hemi-focal, focused) there are technical considerations to consider as well. A hemi-focal approach does not require either TRUS/MRI fusion or MRI-based brachytherapy seed/catheter insertion. The target can be adequately encompassed as the urethra serves as the midline and is clearly defined with a foley catheter and aerated jelly. On the other hand if one considers implanting just the peripheral zone as a hemi-focal technique then using ultrasound alone is likely not sufficient. Other more ultra-focal approaches are even riskier in terms of inadequately encompassing the target and should be done using TRUS/MRI fusion or MRI-based approaches. In fact, some have argued that a disadvantage of radiation therapy for focal therapy is the fact that there is no real time feedback of tissue destruction.
There are additional considerations with defining targets based on mp-MRI for focal therapy. mp-MRI shows a lesion in about 84 % of cases (68, 89, and 100 % of patients with NCCN low, intermediate, and high risk disease) (Kamrava et al. 2013). This means approximately 30 % of low risk patients will not have an identifiable target for an ultra-focal approach. When patients do have a lesion it still needs to be biopsied and cannot be assumed to be cancer. A modified version of the European Society of Uroradiology guidelines has been used to stratify the suspicion of cancer for target lesions. Based on TRUS/MRI fusion biopsies, patients with a cancer suspicion score of 5 have a 94 % chance of having prostate cancer but only 29 % of low risk patients have a score of 4–5 (Kamrava et al. 2013; Sonn et al. 2013). So there will be many low risk patients with either no target lesion or a lesion with a low suspicion score. The effect of hormone therapy on the conspicuity of MRI target lesions also needs to be considered as hormones can change the appearance of targets (Groenendaal et al. 2012).
The best radiation technique (low dose rate seeds, high dose rate brachytherapy, stereotactic body radiation therapy, intensity-modulated proton therapy) for focal therapy is also unknown. When using a low dose rate approach the above consensus statement suggests a preplanned approach that ideally includes mp-MRI fusion with TRUS. Real time intraoperative dose planning is also ideal. In deciding between 125I, 103Pd, and 131Cs it was felt 125I has the most favorable characteristics. Stranded or linked seeds were recommended in the periphery with loose seeds centrally to provide more flexibility. Lower seed activity (~0.5 U) was also felt to be advantageous as it allows for seeds to be spaced closer together.
There are no consensus guidelines for other radiation techniques but there is one dosimetry paper that has examined the magnitude of dose reduction with standard high dose rate brachytherapy whole gland versus hemi-gland treatment (Kamrava et al. 2013) (Fig. 2). 10 whole-gland high dose rate prostate implants were used to generate 10 whole-gland and 20 hemi-gland (consisting of left and right) treatment plans using Inverse Planning Simulation Annealing using Oncentra Masterplan (Nucletron). The hemi-gland contour was a modification of the whole gland contour whereby the urethra was used to divide the volume into a left and right hemi-gland. Hemi-gland treatment decreased the Davg to the rectum, bladder, and urethra by a difference of 7.0, 5.9, and 16.3 %. Another dosimetric consideration with radiation therapy which is not true with other ablative technologies is the fact that the dose of radiation therapy does not fall off immediately outside of the target. This “spill dose” may be advantageous if this dose is adequate to treat insignificant disease that falls within this “spill dose” region but may be a disadvantage when considering retreatment options. In the above-mentioned HDR dosimetry study the “spill dose” was determined for one case and it was determined that the V50 to the contralateral gland was 40 % (Kamrava et al. 2013). This means that if one were to retreat the contralateral hemi-gland and sum the dose from the original plan and the retreatment plan then one would overdose the organs at risk (Kamravaet al. 2013). A modified contour is necessary with a modified dose to meet current dose constraints (Kamrava et al. 2013). The example presented in this dosimetric study did not consider issues such as prostate deformation and size changes following treatment and so the reality of combining dose from an initial implant and retreatment will certainly be more complicated.
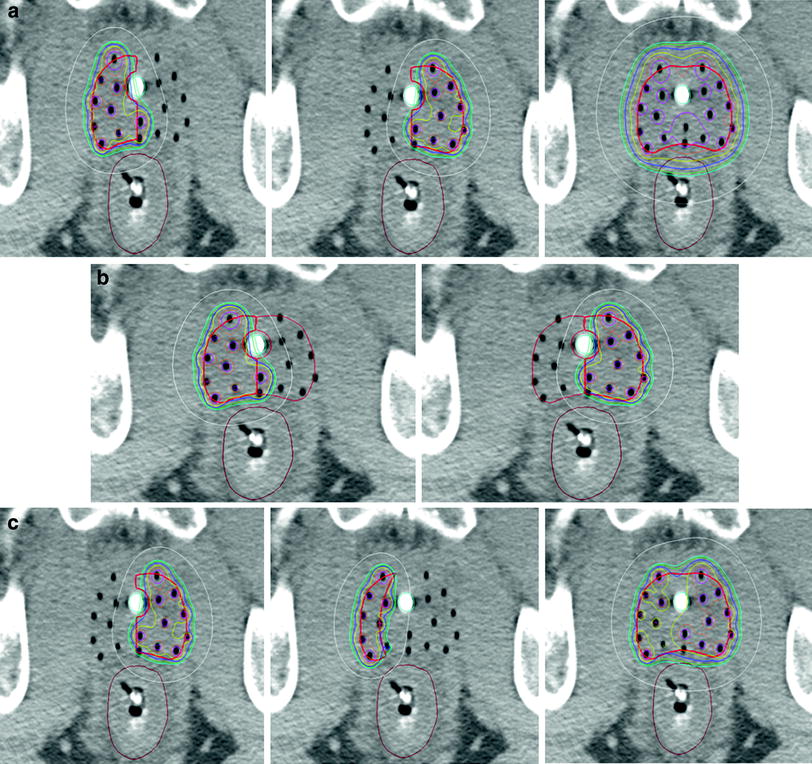

Fig. 2
Examples of the isodose distribution (blue = 100 %, yellow = 110 %, white = 50 %) with high dose rate brachytherapy hemi-gland treatment. These images are axial slices of a high dose rate brachytherapy implant where the whole prostate gland contour was split into right and left hemi-gland contours with the urethra serving as the midline. Figure 2a demonstrates the isodose distribution of a right hemi-gland treatment, a left hemi-gland treatment, and a summation of the right and left hemi-gland plans demonstrating that simply adding the two hemi-gland plans overdoses normal tissues. Figure 2b shows the extent of “spill” from a right and left hemi-gland treatment into the contralateral hemi-gland. Figure 2c demonstrates an example of a left hemi-gland treatment, a matching field in the event of a contralateral failure, and a summation of the two plans showing acceptable doses to organs at risk
5 Clinical Outcomes with Focal Therapy
There is a growing body of focal therapy clinical data. Trying to draw definitive conclusions from the existing literature is challenging. Studies vary greatly in inclusion and evaluation criteria, treatment technique, endpoints, definition of failure, posttreatment assessment protocols, and measures of toxicity. A great deal of insight can still be gleaned, though, from the existing literature. The largest study to date investigating focal therapy was recently updated by Nguyen et al. (Nguyen et al. 2012). 318 patients were treated using intraoperative MRI guidance to deliver low dose rate brachytherapy using 125I (minimum dose 137 Gy) to the peripheral zone only. Entry criteria included being T1c, having a PSA < 15 ng/mL and a biopsy Gleason score 3 + 4 or less. 88 % of patients had Gleason score 3 + 3 and 83 % of the patient cohort was low risk. With a median follow-up of 5.1 years 91.5 % of patients had PSA control by nadir + 2 and 78.1 % at 8 years. Nadir + 2 for low risk patients at 5 and 8 years was 95.1 % and 80.4 % but this improved to 95.6 % and 90.0 % using nadir + 2 and PSA velocity >0.75 ng/mL per year. For intermediate risk patients PSA failure-free survival was 73 % at 5 years and 66.4 % at 8 years. When looking at the cohort of 36 patients who failed by the nadir + 2 definition 16/22 with a PSA velocity >0.75 ng/mL per year had a suspicious lesion on MRI that was biopsy positive for a local recurrence (Gleason 3 + 3 in 5, Gleason 3 + 4 in 2, Gleason 4 + 3 in 2, Gleason 4 + 4 in 1, Gleason 3 + 5 in 1, and Gleason 4 + 5 in 2). For the 10 patients with nadir + 2 failure but PSA velocity <0.75 ng/mL per year only 2 had suspicious lesions on MRI. Both underwent 12 core biopsy and one Gleason score 3 + 3 = 6 disease was found. Based on this data PSA velocity greater than 0.75 ng/mL per year in addition to nadir + 2 better predicts failure in this less than whole gland treatment setting. While this provides some evidence-based guidance, PSA kinetics post partial gland therapy are ultimately not well understood. Our limited understanding of PSA changes post partial gland treatment serves as an impediment to defining ideal follow-up and definitions of failure.
Other focal therapy radiation approaches have used intensity modulated radiation therapy to spare the urethra in a more focused therapy approach. A randomized phase II study of urethra sparing treatment versus standard whole gland therapy for NCCN low risk patients was recently published (Vainshtein et al. 2012). The prescription dose was 75.6 Gy and for the urethral sparing plans the mean proximal and distal urethral doses were limited to 65 Gy and 74 Gy, respectively. Patients had to have no visible lesion within 5 mm of the prostatic urethra seen on MRI. The primary endpoint was a change in urinary health related quality of life at 3 months using the Expanded Prostate Cancer Index (EPIC) quality of life questionnaire. 16 patients were randomized and the trial was subsequently halted as no significant differences in EPIC urinary health related quality of life at 3 months was observed. At a median follow-up of 4.7 years three patients had PSA failure in the urethral sparing group but there were none in the standard treatment group. Two out of the three patients with PSA failure had biopsy proven local failure contralateral to the original site of disease. It is likely that using a 1.5T MRI with no multiparametric sequences that the MRI understaged some of these patients.
Outside of radiation therapy there are a number of important focal therapy studies. HIFU and cryoablation are the most developed techniques, however, multiple other techniques are emerging (Bozzini et al. 2013). The most important non-radiation data comes from a group in the United Kingdom that focuses on the use of HIFU (Ahmed et al. 2011). They conducted a Phase I/II trial in men with either low or intermediate risk disease (PSA ≤ 15, Gleason score ≤4 + 3, stage ≤T2b). They had to have unilateral disease as assessed by TRUS guided biopsies, mp-MRI, and TPM biopsies. Hemi-gland treatment with the urethra defining the mid-gland was delivered using the Sonablate 500. The trial was powered to see if focal therapy significantly reduces the risk of erectile dysfunction at 12 months post-treatment. 20 patients were enrolled and 75 % of them had D’Amico based intermediate risk disease. mp-MRI at 6 months showed residual cancer in the treated lobe in 2 men but no suspicious lesions in the untreated lobe in any. There was an 80 % decrease in PSA seen at 3 months that persisted at 12 months (7.3 vs. 1.5 ng/mL). 2 patients with positive mp-MRI at 6 months had biopsies. Both had low volume disease with 1 mm Gleason 3 + 3 in 1 of 4 and 1 of 5 biopsies. One patient elected to undergo active surveillance and the other was retreated with HIFU. 89 % of patients achieved the trifecta status of pad-free, leak-free continence, and erections sufficient for intercourse. This same group subsequently published the first study using an ultra-focal HIFU approach (Ahmed et al. 2012). Low risk patients (PSA ≤ 15, Gleason score ≤4 + 3, stage ≤T2a) were included and evaluated using 1.5T mp-MRI and transperineal template mapping biopsies. The edges of the ablative zone needed to be at least 10 mm from a neurovascular bundle or at least 5 mm from both neurovascular bundles if disease was bilateral. Untreated areas could not have any histological evidence of prostate cancer. A maximum of two areas could be treated and were treated with at least 3–5 mm margins. Primary outcomes were feasibility, patient acceptability, and side-effect profile. Secondary outcomes were histological and imaging measures of cancer control. 41 men were treated with 49 % receiving unilateral one area ablation, 37 % receiving bilateral two area ablation, and 15 % receiving a midline one area ablation. Median PSA level at baseline was 6.6 ng/mL and at 12 months follow-up was 1.9 ng/mL. 39 patients had a biopsy at 6 months with a mean of 6 cores taken. mp-MRI at 6 months showed signs of residual cancer in the treated areas in nine men and was confirmed on biopsy in seven of them. Two men had negative mp-MRIs but positive biopsies. Both demonstrated clinically insignificant disease. Of the men with positive biopsies five chose active surveillance and four chose retreatment with HIFU. Of the 31 men with good baseline function 84 % achieved the trifecta status of being leak-free, pad-free, erections sufficient for intercourse, and with no evidence of clinically significant disease on mp-MRI at 12 months. Cryotherapy is another very common focal therapy technique and the largest cohort of patients treated with this modality was reported from the Cryo On-Line Database (COLD) registry (Ward and Jones 2012). Focal therapy to a portion of the gland was performed in 1,160 patients (47 % low risk and 41 % intermediate risk) with biochemical control (old ASTRO definition) at 36 months of 75.7 %. This is similar to the biochemical recurrence-free survival of patients treated with whole gland cryotherapy treatment at 75.1 %. Toxicity rates were either similar or better in the focal versus whole gland cryotherapy group: urinary incontinence 1.6 versus 3.1 %, new-onset erectile dysfunction 41.9 versus 67.6 %, rectourethral fistula 0.1 versus 0.4 %, urinary retention 1.2 versus 1.6 %. Definitive conclusions from this data are limited because of the lack of defined criteria for treating patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree