7
Thoracic Brachytherapy
Keyur J. Mehta, Nitika Thawani, and Subhakar Mutyala
LUNG CANCER
In 2015, there will be an estimated 221,200 new cases of lung cancer in the United States. With more than an estimated 158,000 deaths from this disease, it remains the leading cause of cancer deaths (1). Lung cancer has had a long history with brachytherapy, from Yankauer inserting radium-226 (226Ra) into lung tumors in 1922 (2) and Graham and Singer placing radon-222 (222Rn) needles into lung tumors in 1933 (3). Currently, brachytherapy is widely used for lung cancer and at several stages. Following are the uses of interstitial brachytherapy for early stage and locally advanced lung cancer, intraoperative radiation, and intraluminal brachytherapy for endobronchial lesions and palliation.
Early-Stage Disease
Surgery for early-stage lung cancer is the gold standard for definitive treatment. The recommended surgery has always been lobectomy or pneumonectomy. The Lung Cancer Study Group showed that a limited resection had decreased local control as compared to a lobectomy (4). However, a large resection requires the patient to have a reasonable FEV1 (0.8−1.2 L) and a ventilation-perfusion scan corresponding to adequate breathing in other segments. Patients who have long histories of smoking commonly fail to have this lung reserve to handle a large resection. An alternative technique is to perform a sublobar resection with the placement of radioactive seeds at the resection margin. There are several reports of wedge resection for Stage I tumors with placement of the iodine-125 (125I) seed at the resection margin (5–7). This can be done to perform surgery on patients who cannot undergo a lobectomy or pneumonectomy.
Patient selection has much to do with the success of this technique. Studies have found that the size of the tumor, location, and limited resection technique (wedge vs. segmentectomy) also influence the outcome (8,9). In the phase III trial of the American College of Surgeons Oncology Group (ACOSOG; Z4032) comparing sublobar resection versus sublobar resection with brachytherapy, the study authors found that brachytherapy was not associated with increased morbidity and could be performed safely (10,11). Initial reports in abstract form also showed similar rates of local control with sublobar resection alone versus sublobar resection with brachytherapy. They did find, however, that the surgical technique of a wedge resection yielded smaller parenchymal margins, lower yield of lymph node sampling, and lymph node upstaging compared to a segmentectomy (12).
Until more information from the ACOSOG and other studies are available we caution the practitioner in making a treatment decision and to tailor the treatment to each individual. A more extensive surgical resection may not warrant brachytherapy, whereas a more limited procedure may need additional therapy. Ideally patients treated with this technique should be studied in a clinical trial. Currently, there is an ACOSOG Z4099 ongoing trial comparing sublobar resection with or without brachytherapy versus stereotactic body radiation therapy for early-stage lung cancer.
In addition to the larger study group trials listed earlier, these studies showed promising results with planar implants. A large published series comes from Allegheny General Hospital (13). A retrospective series of 101 patients with sublobar resection and seeds placed at the suture line were compared with 102 similar patients with sublobar resection alone. Patients were surgically resected using the video-assisted thoracoscopic surgery (VATS) approach. The implants were made on a Vicryl mesh and planned with a dose of 100 to 120 Gy at a 0.5 cm distance from the plane. The mesh was then sutured to the staple line. The local relapse rates are 2% for seeds (at 18 months) versus 18.6% for sublobar resection alone (at 24 months) (P = .0001). Age and FEV1 were similar in both groups, but the group with the implants had more stage IB patients than the surgery-alone group. Overall, the 4-year survival rates were 60% and 67% for surgery alone and surgery plus implant, respectively, but not significantly different. Published data of longer follow-up (7,14,15) confirm the long-term disease-free and overall survival of these patients.
New England Medical Center and Tufts University has another series (16) of 33 patients who underwent a wedge resection (or segmental resection) and implant. The technique varied slightly, with implanting the strands of the seed directly on the suture line, without the mesh. The dose intended was 125 to 140 Gy at 1 cm depth. The results showed 2/33 (6%) recurrence at the suture line (median follow-up: 51 months) with a 5-year projected survival of 47%. The cancer-specific 5-year survival was 61%.
A newer technique employs the microprecision movement of the da Vinci robotic system. Pisch (17) describes the resection of small tumors with a wedge resection and implantation of the 125I seeds using the da Vinci robot, to assist in fine movements and distances in the chest. In 2010, Blasberg et al reported their results on 11 patients with 12 primary tumors with robotic sublobar resection and 125I seed placement (18). They showed excellent coverage of the tumor bed (V87 = 88.2%, V100 = 84.1%), and low perioperative morbidity, concluding that this technique is a feasible and minimally invasive approach to limited resections.
Planar Seed Implant Technique
After a wedge or sublobar resection, the length and width of the area at risk should be measured. These will be the dimensions of the implant. The implant is composed of 125I in Vicryl suture, called Seed-in-Carrier, with 10 seeds spaced at a 1 cm distance. These sources are commercially available from Oncura, Inc. (Arlington Heights, IL). Each seed is 0.7 mm by 4 mm. After the “at-risk” area is measured out, a custom cutout of an absorbable suture (either Dexon or Vicryl) in mesh form is made. Usually, another centimeter of mesh in all dimensions is needed to suture the implant in place. The area should be drawn with parallel lines longitudinally with 0.7 to 1.5 cm spacing (Figures 7.1−7.6). The exact spacing between the sutures of the seed should be based on the activity of the seeds being implanted. Nomograms from the University of Pittsburgh (Figure 7.7) and Memorial Sloan-Kettering Cancer Center (MSKCC; Figure 7.8) help assist the physician to prospectively plan the dosimetry on the basis of the implant size and the activity of the seeds. The sources should be stitched into the mesh following the lines drawn, remembering that the suture should only be handled with forceps. The suture should be anchored on either side with a small staple and any excess sources on the suture should be cut and disposed of properly, according to radiation protection guidelines. The custom mesh should be placed in the at-risk area and sutured into place, with care taken to not puncture a seed. Alternatively, a mesh fabricated with radioactive seeds is also commercially available (Oncura, Inc.). After the operation is complete and the patient is stable (can be a future date), a CT scan should be taken through the area, with dosimetric planning to follow. This will verify and document the dose that the patient will receive.
Figure 7.1 Using a sterile technique, the suture seed carrier is stitched into the absorbable mesh along grid lines drawn to control the distance between the strands. Note the careful use of long instruments. This implant is 15 × 9 cm (160 seeds) to cover a target area of 13 × 7 cm with 1 cm margin. Ten seeds will cover 9 cm.

Figure 7.2 The completed implant can be trimmed to match the operative bed.

Figure 7.3 A smaller implant 9 × 6 cm (70 seeds) to cover a 7 × 4 target area with 1 cm margin. The sutures are passed through the mesh at least four times per strand and secured with small surgical clips.
Figure 7.4 The suture strand is pulled through a mesh along the grid line. Clips are applied at each end to secure the strands in mesh.

Figure 7.5 An even smaller implant (40 seeds) 7 × 4.5 cm to cover a target area of 5 × 3 cm can be made by doubling back to the next grid line with the same strand. Once the seeds are removed from the steel shield, the implant can be placed in a steel basin at the back of the table to minimize the radiation exposure to the operating room staff.
Implanting Tumor
If the patient cannot undergo surgery, the tumor itself could be implanted. Although this technique has more inferior results compared to surgery, for patients who cannot undergo any surgical resection, this might be the only option to increase dose. The Norris Cancer Center (19) describes volume implants on 14 patients. All patients had lymphatics surgically staged. Iodine-125 was used to implant the tumor. There was a 71% local control rate with 15 months’ median follow-up. All the relapses were in patients who had a Stage III tumor. The dose delivered was 80 Gy at the periphery with a high dose of 200 Gy in the center of the tumor. There was no incidence of radiation pneumonitis.
The tumor and any gross disease should be implanted with radioactive seeds, usually 125I. A needle must be inserted into the tumor and then seeds dropped, either individually or in a line. This technique is called a volume implant. The seeds must be placed to cover a volume of disease, as opposed to the prior technique, a planar implant. MSKCC described this technique (20) with 65% locoregional control.
Figure 7.6 The last implant being sewn in with absorbable sutures over the target area previously agreed on and measured out by the surgeon and the brachytherapist.
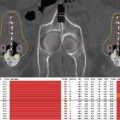
Table 2. Seed activity look-up table | ||
1.0 cm × 1.0 cm | 100 Gy | 120 Gy |
Size | Dose rate @ 0.5 cm | Dose rate @ 0.5 cm |
7 × 7 | 0.38 mCi/seed | 0.46 mCi/seed |
8 × 8 | 0.37 mCi/seed | 0.44 mCi/seed |
9 × 9 | 0.37 mCi/seed | 0.44 mCi/seed |
10 × 10 | 0.36 mCi/seed | 0.43 mCi/seed |
|
|
|
1.2 cm × 1.0 cm | 100 Gy | 120 Gy |
Size | Dose rate @ 0.5 cm | Dose rate @ 0.5 cm |
7 × 7 | 0.42 mCi/seed | 0.50 mCi/seed |
8 × 8 | 0.41 mCi/seed | 0.49 mCi/seed |
9 × 9 | 0.41 mCi/seed | 0.48 mCi/seed |
10 × 10 | 0.41 mCi/seed | 0.47 mCi/seed |
|
|
|
1.5 cm × 1.0 cm | 100 Gy | 120 Gy |
Size | Dose rate @ 0.5 cm | Dose rate @ 0.5 cm |
7 × 7 | 0.49 mCi/seed | 0.59 mCi/seed |
8 × 8 | 0.48 mCi/seed | 0.58 mCi/seed |
9 × 9 | 0.48 mCi/seed | 0.58 mCi/seed |
10 × 10 | 0.47 mCi/seed | 0.57 mCi/seed |
Figure 7.7 Nomogram from the University of Pittsburgh for permanent planar 125I seed dosimetry.
Figure 7.8 Lowell Anderson Nomogram from Memorial Sloan-Kettering Cancer Center for 125I permanent implants seed dosimetry.
More recently, Wang et al describe a volumetric implant using the technique of CT-guided placement of radioactive 125I seeds (21). A preprocedural plan was constructed on three-dimensional (3D) imaging to estimate the number of seeds to be used and their optimal distribution. A total of 21 patients were treated in this manner, all under local anesthesia. They reported a pain response rate in 83.3%. A tumor response rate was considered as a partial or complete response on serial postimplant imaging and was calculated to be 71.4%. Local tumor control rate was 85.7%. The procedure was well tolerated and the implant was successful. Only six patients died of progression of the primary tumor. The CT-guided implant certainly has its advantages of obviating general anesthesia, and should be explored further.
Locally Advanced Disease
Surgical Limitations
Certain tumors are deemed unresectable or marginally resectable on the basis of anatomic locations, such as proximity to bones or great vessels or superior sulcus tumors. Brachytherapy can assist in converting an unresectable, or marginally resectable, tumor into an acceptable oncological resection. Retrospective series from MSKCC (22) showed that Stage III patients with mediastinal involvement had similar median survival (16 vs 17 months) and a 5-year survival (15%) for complete resection versus incomplete resection plus brachytherapy, which were both better than no resection and brachytherapy alone or no resection and no brachytherapy. In another series with all lung cancer stages, there was an increase of 50% (8 to 12 months) in medial survival with brachytherapy after incomplete resection compared with no surgery (23). This was compared with a 17 month median survival for complete resection.
New York Hospital (24) looked at this technique in a prospective study. Twelve patients with Stage III non-small cell lung cancer (NSCLC) who had gross or microscopically positive margins after resection were implanted with a planar implant. The implants were composed of either 125I or palladium-103 (103Pd) embedded in a Gelfoam plaque. The dose prescribed was to a 1 cm margin around the area of positive margin. All patients received either preoperative or postoperative external beam, from 45 to 60 Gy. The results showed 82% local control with the addition of brachytherapy for positive margin after surgery. The 2-year overall and cancer-specific survivals were 45% and 56%, respectively. MSKCC (25), in another series, also reported a 75% locoregional control with partial resection and implant, compared with an 86% locoregional control with full resection.

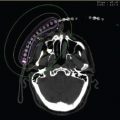
Figure 7.9 Dosimetry of a planar seed implant for a superior sulcus tumor.
Planar Seed Placement
After maximal resection by the surgeon, the area at risk (close or positive margin) must be noted by the radiation oncologist and surgeon. The area should be measured, usually adding 0.5 to 1 cm to all dimensions for a radiation dosimetric margin. The geometry of the implant could take any shape; however, a rectangle is the easiest to make and to perform dosimetry. One way to clear this margin is to place a permanent planar implant with interstitial seeds. The description on how to fashion an implant and how to place was given earlier. Toxicity from this type of implant is low, with 7.9% grade 3 to 4 toxicity defined as requiring surgical intervention (26). The specific toxicities were hydropneumothorax, radiation pneumonitis, and esophageal fistulas. The esophageal fistulas were from placing the implant on an esophagus that had been surgically violated, and the muscular layer was not of full thickness (27). The dosimetry of a planar seed implant for a superior sulcus tumor is pictured in Figure 7.9.
Afterloading Catheters and Intraoperative Radiation Therapy
Another option to treat a close or positive margin after resection for locally advanced disease is by placing temporary afterloading catheters or with intraoperative radiation therapy (IORT).
Afterloading Catheters
The group at Cedars-Sinai Medical Center reported its results of a wedge resection with high dose rate (HDR) remote afterloading brachytherapy (6). They treated patients twice daily with HDR brachytherapy for seven fractions before removing the catheters, to a median dose of 24.5 Gy. In the follow-up period up to 27 months there were only four recurrences. Complications included prolonged air leak, atrial fibrillation, pneumonia, trapped lung, empyema, bleeding, and recurrent laryngeal nerve injury. Given the aforementioned complication risk, and the risk of movement and kinking of the catheters, this technique is typically not recommended unless radioactive seed implantation or IORT is not available.
Afterloading involves placing hollow blind-ended plastic catheters along the area at risk. The catheters should be spaced out by 1 cm in parallel lines. The open end of the catheter should be directed out of the skin through the surgical wound or percutaneous sites adjacent to the surgical incision. Care must be taken to not kink the catheters in any sharp angles, as this would not allow proper loading of the catheters. After appropriate time is given for the patient to stabilize, the patient should have a CT-generated treatment plan in the radiation department. Afterloading can be treated with low dose rate (LDR), pulsed dose rate (PDR), or HDR. LDR, a less common form of treatment now, requires the patient to have active radioactive sources on a string to be placed into the catheters and remain for a few days. During the interim, the patient must be isolated in a radiation safe room, with full radiation precautions. The radiation and catheters are removed at the appropriate time and the patient can be removed from radiation precautions. MSKCC reported implanting the mediastinum with afterloading techniques (LDR) with a good local control and 2-year actuarial survival (76% and 51%, respectively) for N2 disease (20).
HDR and PDR treatments could be performed with an afterloader, such as Nucletron Co. (Veenendal, the Netherlands) microSelectron or a similar device. The patient is implanted with the same catheters as mentioned in the preceding text. The radiation is delivered with only one source, which is computer controlled and can be placed at various positions and dwell times. This flexibility allows for more dose conformality than LDR. Also, all treatment is delivered in a shielded room, eliminating the dose to staff.
Intraoperative Radiation Therapy
Several series have been published, describing this technique. The group from University Clinic of Navarra, Pamplona, Spain, reported early clinical results of a phase I to II trial of IORT for Stage III lung cancer (28). Eligible patients had unresectable hilar tumors, or developed residual hilar, mediastinal, and/or chest wall disease after resection. They treated 34 patients with resection, if feasible, IORT of electron beams of 10 to 15 Gy in a single fraction, followed by external radiotherapy from 46 to 50 Gy over 5 weeks. Freedom from thoracic recurrence was 30%, 65% in cases of tumor resection, in a median follow-up time of 12 months. One patient suffered from a bronchopleural fistula, and another patient developed severe hemoptysis. Other less severe toxicities included acute pneumonitis and esophagitis, and late lung fibrosis.
Another pilot study from the group at Universiy Medical School in Graz, Austria, reported its results with single-fraction IORT (10−20 Gy using 7 to 20 MeV electrons) followed by external radiation therapy (46 Gy in 23 fractions) for unresectable NSCLC (29). Thirty-one patients were treated, 23 being evaluable for the study. Thirteen patients had a complete response, eight had at least a 50% response, and two had less than 50% response. Two patients had a local recurrence, one had local and distant recurrence, and two had distant recurrence only. The recurrence-free survival rate was 53.2% at the time of analysis.
Aristu et al, from the Pamplona, Spain, group mentioned previously had later published an important study emphasizing the multimodality approach for locally advanced lung cancer, incorporating IORT as part of the treatment regime (30). Sixty-two patients with Stage IIIA and IIIB NSCLC were treated with neoadjuvant cisplatin, mitomycin, and vindesine (median: 3 cycles), followed by surgical resection. Single-fraction IORT (10−15 Gy) was delivered at the time of surgery, later followed by external radiotherapy (46 Gy in 23 fractions) 4 weeks after surgery. Of the 55 evaluable patients, 29 patients underwent resection. Complete resection was achievable in 12 of 14 (85%) Stage IIIA patients and in only 6 of 15 (40%) Stage IIIB patients. There was no residual tumor found in three of the 29 patients. Median survival time was 10 months. Five-year survival rates were 29% and 7% in Stages IIIA and IIIB respectively.
For IORT, more equipment is needed. The radiation can be delivered using a mobile accelerator into a shielded operating room or in the radiation department, where a radiation vault is also a functional operating room. The surgeon demarcates the area at risk. The normal tissue can be moved out of the field or shielded with thin strips of lead. Either the cone from the linear accelerator is inserted into the patient or an applicator for HDR brachytherapy is placed, such as the Harrison–Anderson–Mick applicator (HAM applicator; Mick Industries, Westchester, NY). All personnel must leave the room before the radiation is delivered, which usually lasts only a few minutes.
Complications
Complications from any of these techniques are similar and minimal compared to the surgery itself. There could be some instances of poor wound healing or abscess formation, although very rare. The most concerning toxicity would be fistula formation or hemorrhage of large vessels. Intact tissue can tolerate the very low dose rate (VLDR) radiation well (31); however, any injury, either by tumor or surgery, can predispose to a fistula or hemorrhage. Care must be used to avoid placement of the seeds or catheters directly on any injured critical organ at risk, such as the esophagus (27) or blood vessels. This can be avoided if an implant is necessary by adding another layer of luminal protection for the vessel of the esophagus, by biological or artificial technique.
ENDOBRONCHIAL LESIONS
Endobronchial Primary
Endobronchial brachytherapy (EBBT) has been used alone or as a boost in addition to external beam radiotherapy (EBRT) for definitive treatment. One pilot study showed results for small superficial lesions limited to the bronchus treated with HDR EBBT only (32). The treatment dose was five fractions of 7 Gy at 1.0 cm depth. The 2 year actuarial survival was 58%, but with two of 19 deaths from late toxicity (hemoptysis).
Marsiglia et al described their experience in treating 34 patients with non-small cell bronchial carcinoma with EBBT only, as the patients were ineligible for surgery or EBRT (33). The treatment prescription was 30 Gy in six fractions. Local failure occurred in five patients (15%). At a 2-year median follow-up, the local control rate was 85%, and the survival rate was 78%. There was one patient who suffered from a pneumothorax, but had no other severe treatment-related toxicities.
A more recent study reported results on 106 patients treated with HDR EBBT who were not eligible for surgery or EBRT either due to respiratory insufficiency, previous EBRT, or recurrence after surgery (34). Six fractions of 5 to 7 Gy were prescribed 1 cm from the source. At 3 months, there was a complete response rate of 59.4%. At 3 and 5 years, the local control, overall survival, and cause-specific survival rates were 60.3% and 51.6%, 47.4 and 24%, and 67.9 and 48.5%, respectively. Despite these promising results, five patient deaths (two from hemoptysis, three from bronchial necrosis) were attributed to EBBT.
A phase II study by Anacak et al evaluated EBBT as a boost to EBRT. They treated 30 patients with Stage III NSCLC with 60 Gy EBRT and 15 Gy in three fractions of HDR EBBT (35). They found a 76.7% tumor response rate, and excellent palliation of cough, hemoptysis, chest pain, and dyspnea. However, median locoregional disease-free survival was 9.6% at 5 years. Acute side effects included radiation bronchitis in 70% and esophagitis in 6.6% of patients. Late side effects included bronchial fibrosis in 25%, esophageal fibrosis in 12.5%, and fatal hemoptysis in 10.5%. This shows that the addition of endobronchial radiation to EBRT decreases the symptoms without any change in the overall survival.
Palliation
One of the most common uses of brachytherapy is EBBT for palliation. Patients with lung disease can get obstructive pneumonia, hemoptysis, or both. These symptoms can drastically affect the quality of life or can even be life-threatening. Radiation therapy can be administered for palliation, either with external beam or with brachytherapy. Brachytherapy provides a benefit as higher doses could be directly given to the tumor, sparing normal lung. The main disadvantage of brachytherapy would be subjecting the patient to a procedure to insert the catheter, which some end-stage patients may not be able to tolerate. The ABS recommends a selection process for patients to receive brachytherapy as shown in Table 7.1.
Experience
A comparison was made between EBRT and EBRT plus endobronchial radiation (36). This was carried out in a randomized fashion with 95 patients. The endobronchial treatment was two 7.5 Gy fractions 1 week apart. The EBRT was 30 Gy in 10 fractions or 60 Gy in 30 fractions. The results showed added benefit with endobronchial therapy by increasing the incidence of re-expansion and decreasing the incidence of dyspnea, along with prolonging the duration of palliation. The toxicity was low in either arm, 13% versus 15% of massive hemoptysis.
Another randomized study compared EBRT with EBBT (37). The doses were 30 Gy in 10 to 12 fractions versus one fraction of 15 Gy at 1 cm by HDR. The results showed a better relief of symptoms with EBRT versus brachytherapy, 91% versus 76%, respectively. There was also a modest improvement in survival, 287 versus 250 days, respectively. Also, more patients who received brachytherapy required EBRT (51%) later compared with patients who received EBRT requiring brachytherapy (28%) later. The toxicity profiles were identical.
The MD Anderson Cancer Center published the 10-year experience with EBBT for palliation (38). There were 175 patients, 160 of whom had received previous EBRT. The treatment regimen was 15 Gy in two fractions at 6 mm from the catheter for a total of 30 Gy. Results showed 66% subjective improvement (34% slight improvement and 32% significant improvement) and 78% objective improvement on repeat bronchoscopy. The complications were 11% with massive hemoptysis at 5%. Table 7.2 is a summary of the published series on endobronchial brachytherapy.
Table 7.1 American Brachytherapy Society’s recommendations for endobronchial brachytherapy
• Patients with significant endobronchial tumor component, causing symptoms such as shortness of breath, hemoptysis, persistent cough, and signs of postobstructive pneumonitis. Tumors that protrude into the lumen are considered suitable, as opposed to extrinsic tumors that compress the bronchus or the trachea. The catheter should be able to pass into (and preferably past) the obstructed bronchus. Endobronchial brachytherapy can generally give a quicker palliation of obstruction than EBRT. Furthermore, brachytherapy can be more convenient compared to 2 to 3 wk of daily EBRT for many patients. • Patients who do not undergo resection because of poor lung function or distant metastasis. • Patients who, because of poor lung function, are unable to tolerate any external irradiation. • Patients with previous EBRT of sufficient total dose to preclude further EBRT. • Patients with sufficient life expectancy (usually > 3 mo) to benefit from palliation. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree